
Chemistry, 27.07.2019 02:30 Rockey3876
How many grams of ice would be melted by the energy obtained as 16.1 g of steam is condensed at 100°c and cooled to 0°c? specific heat (ice) = 2.10 j/gºc specific heat (water) = 4.18 j/gºc heat of fusion = 333 j/g heat of vaporization = 2258 j/g 43.1 kg 36.4 kg 129 g 6.73 kg 20 g

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 16:00
Measuring which physical property is most likely to produce the most precise results when trying to identify a substance
Answers: 1

Chemistry, 21.06.2019 16:00
If the same amount of cacl2 is added to equal volumes of water and maple syrup, which will have the higher temperature?
Answers: 1

Chemistry, 22.06.2019 03:50
Which of the following statements about acidic water is true? a. acid has no effect on the h,o molecules. b. the solution contains a larger number of oh ions than h,o ions. c. the solution contains a larger number of h,o ions than qh ions. d. the solution contains an equal number of h,o ions and oh ions. none of the above e.
Answers: 1

Chemistry, 23.06.2019 03:00
Describe the properties of sodium, chlorine, and sodium chloride
Answers: 1
You know the right answer?
How many grams of ice would be melted by the energy obtained as 16.1 g of steam is condensed at 100°...
Questions

English, 10.01.2021 21:10

Mathematics, 10.01.2021 21:10

English, 10.01.2021 21:10


Advanced Placement (AP), 10.01.2021 21:10

Mathematics, 10.01.2021 21:10

Mathematics, 10.01.2021 21:10

Mathematics, 10.01.2021 21:10

Mathematics, 10.01.2021 21:10

Mathematics, 10.01.2021 21:10


English, 10.01.2021 21:10

English, 10.01.2021 21:10



Mathematics, 10.01.2021 21:10




Computers and Technology, 10.01.2021 21:10

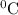
![[16.1\times 2258]J+[16.1\times 4.18\times (100-0)]J](/tpl/images/0137/1545/534f5.png) = 43083.6 J
= 43083.6 J

