
Chemistry, 27.07.2019 03:20 glowbaby123
A25 -ml of 0.30 m of ammonia, nh (a), solution is titrated with 0.15 m hydrochloric acid, hcl (aq calculate the ph of the solution a) determine the volume of the acid required to reach equivalence point. b) at half the equivalence point. c) at the cquivalence point. d) when 55 ml of the hcl has been added. e choose a suitable indicator for this titration.

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 11:50
The chemical bond connecting one nucleotide with the next one along the nucleic acid chain is called a
Answers: 3

Chemistry, 22.06.2019 12:00
What are the first two quantum numbers for the electrons located in subshell 4d? what are the first three quantum numbers for the electrons located in subshell 2s? how many electrons can be held in a sublevel l = 3? how many electrons can be held in the energy level n = 4? how many electrons in an atom can share the quantum numbers n = 4 and l = 3?
Answers: 1

Chemistry, 22.06.2019 15:10
Which statement describes the phase change that occurs when dry ice is placed in an open container at room temperature?
Answers: 1

Chemistry, 22.06.2019 15:30
The reactions of photosynthesis occur in the of plant cell? a.mitochondria. b. lysosomes. c. chloroplasts. d. chlorophyll
Answers: 1
You know the right answer?
A25 -ml of 0.30 m of ammonia, nh (a), solution is titrated with 0.15 m hydrochloric acid, hcl (aq ca...
Questions

Biology, 10.12.2020 14:00

Mathematics, 10.12.2020 14:00


Mathematics, 10.12.2020 14:00

History, 10.12.2020 14:00

Mathematics, 10.12.2020 14:00

Law, 10.12.2020 14:00

History, 10.12.2020 14:00


History, 10.12.2020 14:00





History, 10.12.2020 14:00

Biology, 10.12.2020 14:00

Mathematics, 10.12.2020 14:00

Advanced Placement (AP), 10.12.2020 14:00

Law, 10.12.2020 14:00

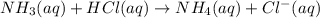
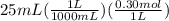
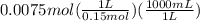
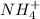 will form and the solution will act as a buffer solution as it has a weak base(ammonia) and its conjugate acid(ammonium ion).
will form and the solution will act as a buffer solution as it has a weak base(ammonia) and its conjugate acid(ammonium ion).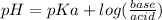
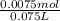
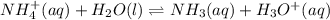
![Ka=\frac{[x][x]}{[0.10-x]}](/tpl/images/0137/3088/1f1fd.png)
![Ka=\frac{[x]^2}{[0.10-x]}Ka for [tex]NH_4^+](/tpl/images/0137/3088/dc53e.png) is
is 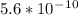 . It's a very low value so the x on the bottom could be neglected and the expression could be written as:
. It's a very low value so the x on the bottom could be neglected and the expression could be written as:
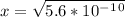
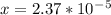
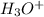 is
is 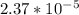 M.
M. 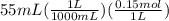
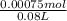 = 0.009375 M
= 0.009375 M

