

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 23:30
Two atoms interact with each other as shown by the equation. complete the equation by filling in the missing parts. 1 2 3 4 5 h he li
Answers: 2

Chemistry, 22.06.2019 00:00
Several kinds of bears are found on earth. most bears are brown or black, but one type of bear, the polar bear, is white. what process led to this difference in fur color? explain your answer.
Answers: 1

Chemistry, 23.06.2019 03:20
What kind of intermolecular forces act between a hydrogen fluoride molecule and a hydrogen peroxide molecule? note: if there is more than one type of intermolecular force that acts, be sure to list them all, with a comma between the name of each force.
Answers: 1

Chemistry, 23.06.2019 06:50
The student repeated the experiment using a higher concentration of acid. the same volume of acid and the same mass of magnesium ribbon were used. what volume of hydrogen gas would have been produced after 60 seconds?
Answers: 1
You know the right answer?
The enthalpy change for converting 10.0 g of ice at -25.0°c to water at 80.0°c is kj. the specific...
Questions

Law, 10.02.2020 21:06

English, 10.02.2020 21:06



Biology, 10.02.2020 21:06


Mathematics, 10.02.2020 21:06


Mathematics, 10.02.2020 21:06


English, 10.02.2020 21:06

Physics, 10.02.2020 21:06


History, 10.02.2020 21:06


Chemistry, 10.02.2020 21:06

Mathematics, 10.02.2020 21:06



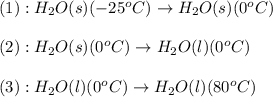
![\Delta H=[m\times c_{p,s}\times (T_{final}-T_{initial})]+n\times \Delta H_{fusion}+[m\times c_{p,l}\times (T_{final}-T_{initial})]](/tpl/images/0137/4660/5cd06.png)
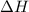 = enthalpy change = ?
= enthalpy change = ?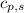 = specific heat of solid water = 2.09 J/gk
= specific heat of solid water = 2.09 J/gk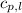 = specific heat of liquid water = 4.18 J/gk
= specific heat of liquid water = 4.18 J/gk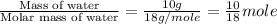
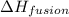 = enthalpy change for fusion = 6.01 KJ/mole = 6010 J/mole
= enthalpy change for fusion = 6.01 KJ/mole = 6010 J/mole![\Delta H=[10g\times 2.09J/gK\times (273-248)k]+\frac{10}{18}mole\times 6010J/mole+[10g\times 4.18J/gK\times (353-273)k]](/tpl/images/0137/4660/7e566.png)
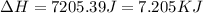 (1 KJ = 1000 J)
(1 KJ = 1000 J)

