

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 04:30
In which phase(s) do the molecules take the shape of the container?
Answers: 1

Chemistry, 22.06.2019 05:30
Liv sheldon given the balanced equation for an organic reaction: c2h2 + 2cl2 → c2h2cl4 this reaction is best classified as *
Answers: 1

Chemistry, 22.06.2019 16:30
Correct relationship between molecular formula and empirical formula
Answers: 1

Chemistry, 22.06.2019 19:00
Which is the solubility product expression for caf2(s)?  [ca2+]/[f–]2  [ca2+][f2–]  [ca]+[f]2  [ca2+][f–]2
Answers: 3
You know the right answer?
At 85°c, the vapor pressure of a is 566 torr and that of b is 250 torr. calculate the composition of...
Questions


Mathematics, 15.04.2021 08:00

English, 15.04.2021 08:00




Mathematics, 15.04.2021 08:00

Mathematics, 15.04.2021 08:00

Mathematics, 15.04.2021 08:00


Mathematics, 15.04.2021 08:00

Mathematics, 15.04.2021 08:00


Mathematics, 15.04.2021 08:00



Biology, 15.04.2021 08:00

Mathematics, 15.04.2021 08:00


Chemistry, 15.04.2021 08:00

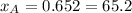 %
%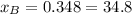 %
%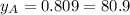 %
%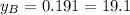 %
%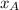 and
and 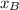 molar fractions can be calculated as:
molar fractions can be calculated as: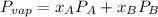
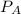 and
and 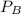 are the vapor pressures of the pure compounds. A substance boils when its vapor pressure is equal to the pressure under it is; so it boils when
are the vapor pressures of the pure compounds. A substance boils when its vapor pressure is equal to the pressure under it is; so it boils when 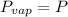 . When the pressure is 0.60 atm, the vapor pressure has to be the same if the mixture is boiling, so:
. When the pressure is 0.60 atm, the vapor pressure has to be the same if the mixture is boiling, so: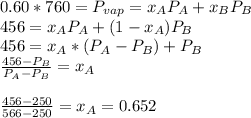
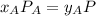 , where P is the total pressure and y is the fraction in the vapor phase, so:
, where P is the total pressure and y is the fraction in the vapor phase, so: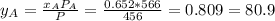 %
%

