
Chemistry, 29.07.2019 19:20 karatekats1
Use the free energies of formation given below to calculate the equilibrium constant (k) for the following reaction at 298 k. 2 hno3(aq) + no(g) → 3 no2(g) + h2o(l) k = ? δ g0f(kj/mol) -110.9 87.6 51.3 -237.1

Answers: 2


Another question on Chemistry


Chemistry, 22.06.2019 05:30
What is the mass defect of a mole of nuclei with 1.8 x 10^15 j/mol binding energy?
Answers: 1


Chemistry, 22.06.2019 11:00
Which statement is true about hcl? (5 points) select one: a. it is a salt because it increases the concentration of metallic ions. b. it is a salt because it is formed by the reaction of an acid and a base. c. it is an acid because it increases the concentration of hydroxyl ions. d. it is an acid because it increases the concentration of hydronium ions.
Answers: 1
You know the right answer?
Use the free energies of formation given below to calculate the equilibrium constant (k) for the fol...
Questions


Chemistry, 05.05.2020 10:20




Biology, 05.05.2020 10:20





Spanish, 05.05.2020 10:20

Mathematics, 05.05.2020 10:20

English, 05.05.2020 10:20

History, 05.05.2020 10:20

Mathematics, 05.05.2020 10:20




Mathematics, 05.05.2020 10:20




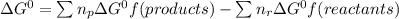
![\Delta G^{0}=[3\Delta G^{0}f(NO2)+3\Delta G^{0}f(H2O)]-[2\Delta G^{0}f(HNO3)+1\Delta G^{0}f(NO)]](/tpl/images/0147/7522/36e5a.png)
![\Delta G^{0}=[3\Delta G^{0}f(51.3)+3\Delta G^{0}f(-237.1)]-[2\Delta G^{0}f(-110.9)+1\Delta G^{0}f(87.6)]](/tpl/images/0147/7522/2150b.png)



