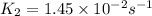Chemistry, 29.07.2019 19:20 faithtunison
The reaction c4h8(g)⟶2c2h4(g) c4h8(g)⟶2c2h4(g) has an activation energy of 262 kj/mol.262 kj/mol. at 600.0 k,600.0 k, the rate constant, , is 6.1×10−8 s−1.6.1×10−8 s−1. what is the value of the rate constant at 785.0 k?

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 15:00
Many ionic compounds and a few highly polar covalent compounds are because they completely ionize in water to create a solution filled with charged ions that can conduct an electric current.
Answers: 1

Chemistry, 22.06.2019 15:30
Count the number of each type of atom in the equation below, and then balance the equation. write in the numbers of atoms and coefficients. add a 1 if there should be no coefficient. cs2(l) + o2(g) → co2(g) + so2(g) c [ ] s [ ] o > c [ ] s [ ] o [ ] cs2(l) + [ ] o2(g) > [ ] co2(g) + [ ] so2(g)
Answers: 3

Chemistry, 22.06.2019 18:30
When a device is used in a circuit in which the voltage is 81 v the current flowing through the device is 3 a what is the resistance of the device
Answers: 2

Chemistry, 22.06.2019 23:10
Match the formula for the following compound: magnesium sulfate heptahydratemgs·7h2omg2so4·7h2omg(so4)2·7h2omgso4·7h2o
Answers: 1
You know the right answer?
The reaction c4h8(g)⟶2c2h4(g) c4h8(g)⟶2c2h4(g) has an activation energy of 262 kj/mol.262 kj/mol. at...
Questions

Mathematics, 04.08.2019 06:30


Mathematics, 04.08.2019 06:30

Chemistry, 04.08.2019 06:30





Social Studies, 04.08.2019 06:30




Mathematics, 04.08.2019 06:30

Biology, 04.08.2019 06:30


Mathematics, 04.08.2019 06:30

Advanced Placement (AP), 04.08.2019 06:30

Biology, 04.08.2019 06:30

Chemistry, 04.08.2019 06:30

History, 04.08.2019 06:30

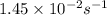
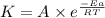
![\log (\frac{K_2}{K_1})=\frac{Ea}{2.303\times R}[\frac{1}{T_1}-\frac{1}{T_2}]](/tpl/images/0147/7591/6d953.png)
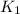 = rate constant at
= rate constant at 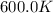 =
= 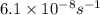
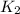 = rate constant at
= rate constant at 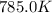 = ?
= ?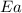 = activation energy for the reaction = 262 kJ/mole = 262000 J/mole
= activation energy for the reaction = 262 kJ/mole = 262000 J/mole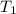 = initial temperature =
= initial temperature = 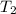 = final temperature =
= final temperature = ![\log (\frac{K_2}{6.1\times 10^{-8}s^{-1}})=\frac{262000J/mole}{2.303\times 8.314J/mole.K}[\frac{1}{600.0K}-\frac{1}{785.0K}]](/tpl/images/0147/7591/a9479.png)