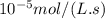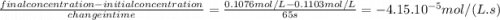Chemistry, 29.07.2019 19:30 tamikagoss22
At the beginning of an experiment, the concentration of nitrogen dioxide in a reaction vessel was 0.1103 mol/l. after 65.0 s, the concentration decreased to 0.1076 mol/l. what is the average rate of decomposition of no2 during this time interval, in mol/(l ∙ s)?

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 22:30
Complete the sentence. the lower the hydrogen ion concentration, the the ph. higher lower closer to 7 closer to 0
Answers: 2

Chemistry, 22.06.2019 03:40
Kc = 0.040 for the system below at 450oc. if a reaction is initiated with 0.40 mole of cl2 and 0.40 mole of pcl3 in a 2.0 liter container, what is the equilibrium concentration of cl2 in the same system? pcl5(g) ⇄ pcl3(g) + cl2(g)
Answers: 3

Chemistry, 22.06.2019 18:00
How does climate change cause the ocean's thermohaline current to slow down?
Answers: 3

Chemistry, 22.06.2019 20:30
We are hoping to create 5.72 grams of glucose. the plant was given 4.75 liters of co2 and 2.81 g of h20. which reactant was the limiting reagent? how much excess mass did we have of the other reactant?
Answers: 3
You know the right answer?
At the beginning of an experiment, the concentration of nitrogen dioxide in a reaction vessel was 0....
Questions

Computers and Technology, 10.04.2021 16:10


Chemistry, 10.04.2021 16:10

Biology, 10.04.2021 16:10

Mathematics, 10.04.2021 16:10


World Languages, 10.04.2021 16:10

English, 10.04.2021 16:10


Advanced Placement (AP), 10.04.2021 16:10

History, 10.04.2021 16:10

Chemistry, 10.04.2021 16:10





Mathematics, 10.04.2021 16:10