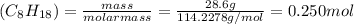Chemistry, 29.07.2019 23:30 Gladistshiala267
Consider the combustion reaction for octane (c8h18), which is a primary component of gasoline. 2c8h18+25o2⟶16co2+18h2o how many moles of co2 are emitted into the atmosphere when 28.6 g c8h18 is burned?

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 13:20
Amixture of gaseous sulfur dioxide and oxygen are added to a reaction vessel and heated to 1000 k where they react to form so3(g). if the vessel contains 0.669 atm so2(g), 0.395 atm o2(g), and 0.0851 atm so3(g) after the system has reached equilibrium, what is the equilibrium constant kp for the reaction: 2 so2(g) o2(g) ⇌ 2 so3(g)
Answers: 3


Chemistry, 21.06.2019 22:30
Which of these properties, used alone, would be least useful in identifying most minerals? a. color b. luster c. streak d. density
Answers: 2

Chemistry, 22.06.2019 01:30
In a spacecraft, the following reaction occurs: co2(g) + 2lioh(s) -> lico3(s) + h2o(i) (i attached picture of equation) how many liters of carbon dioxide will 4 moles of lithium hydroxide (lioh) absorb? (one mole of any gads occupies 22.4 l under certain conditions of temperature and pressure. assume those conditions for this equation.) 45l 6.0l 3.0l 34l
Answers: 1
You know the right answer?
Consider the combustion reaction for octane (c8h18), which is a primary component of gasoline. 2c8h1...
Questions

Mathematics, 29.11.2020 21:10

Mathematics, 29.11.2020 21:10

Mathematics, 29.11.2020 21:10

Mathematics, 29.11.2020 21:10

Physics, 29.11.2020 21:10

Mathematics, 29.11.2020 21:10

Computers and Technology, 29.11.2020 21:10






Business, 29.11.2020 21:10





Mathematics, 29.11.2020 21:10

Mathematics, 29.11.2020 21:10

Chemistry, 29.11.2020 21:10