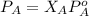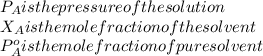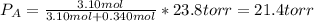Chemistry, 30.07.2019 02:10 keviongardner
If 0.340 mol of a nonvolatile nonelectrolyte are dissolved in 3.10 mol of water, what is the vapor pressure ph2o of the resulting solution? the vapor pressure of pure water is 23.8 torr at 25 ∘c . express your answer with the appropriate units.

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 17:10
A+b→2c when the reaction begins, the researcher records that the rate of reaction is such that 1 mole of a is consumed per minute. after making changes to the reaction, the researcher notes that 2 moles of a are consumed per minute. what change could the researcher have made to effect this change?
Answers: 1

Chemistry, 22.06.2019 02:50
Consider the equilibrium system: 2icl(s) ⇄ i2(s) + cl2(g) which of the following changes will increase the total amount of of cl2 that can be produced? all of the listed answers are correct decreasing the volume of the container removing the cl2 as it is formed adding more icl(s) removing some of the i2(s)
Answers: 1

Chemistry, 22.06.2019 12:30
Suppose you wanted to make 100 grams of water. what is the molar mass of water (h2o)?
Answers: 2

Chemistry, 22.06.2019 15:40
Use the periodic table to complete this equation that represents nuclear fission processesun - ba c 3 n
Answers: 2
You know the right answer?
If 0.340 mol of a nonvolatile nonelectrolyte are dissolved in 3.10 mol of water, what is the vapor p...
Questions

Physics, 14.06.2021 02:10


Spanish, 14.06.2021 02:10

English, 14.06.2021 02:10

Mathematics, 14.06.2021 02:10

Business, 14.06.2021 02:20



Computers and Technology, 14.06.2021 02:20


Mathematics, 14.06.2021 02:20

Geography, 14.06.2021 02:20


Mathematics, 14.06.2021 02:20

Mathematics, 14.06.2021 02:20



Chemistry, 14.06.2021 02:20


Mathematics, 14.06.2021 02:20