

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 20:30
If 10.g of agno3 is available, what volume of 0.25 m agno3 can be prepared
Answers: 1

Chemistry, 22.06.2019 08:00
Identify a strong intermolecular force of attraction between an alcohol
Answers: 1

Chemistry, 22.06.2019 11:30
If we compare and contrast electromagnetic waves with sound waves, all but one statement is true. that is a) sound waves require a medium to travel while electromagnetic waves do not. b) electromagnetic waves can travel through the vacuum of space while sound waves cannot. c) electromagnetic waves must have a medium in which to travel, but sound waves can travel anywhere. eliminate d) sound waves must bounce off of matter in order to travel while electromagnetic waves do not require matter to be present.
Answers: 3

Chemistry, 22.06.2019 16:00
No copying 15 pts how does a free-body diagram tell you about the net force on an object?
Answers: 2
You know the right answer?
Determine the value of kc for the following reaction if the equilibrium concentrations are as follow...
Questions



Advanced Placement (AP), 07.07.2019 19:30

Mathematics, 07.07.2019 19:30


Mathematics, 07.07.2019 19:30

English, 07.07.2019 19:30

Mathematics, 07.07.2019 19:30


History, 07.07.2019 19:30

History, 07.07.2019 19:30



History, 07.07.2019 19:30

Geography, 07.07.2019 19:30




Chemistry, 07.07.2019 19:30

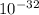
![K_{c} =\frac{[HI]^{2}[Cl_{2}] }{[HCl]^{2}}=\frac{[5.6*10^{-16} ]^{2} [0.0019]}{[0.13]^{2} }=3.53*10^{-32}](/tpl/images/0149/3443/d62e7.png)


