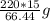Chemistry, 30.07.2019 17:10 celeste961
Achemistry student needs 15.00g of acetic acid for an experiment. he has available 220.g of a 30.2% w/w solution of acetic acid in benzene. calculate the mass of solution the student should use. if there's not enough solution, press the "no solution" button. be sure your answer has the correct number of significant digits. g ×10no solution

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 01:00
What are the variables in gay-lussac’s law? pressure and volume pressure, temperature, and volume pressure and temperature volume, temperature, and moles of gas
Answers: 1


Chemistry, 22.06.2019 21:30
In science class richard learns that a substance has a boiling point of 230 fahrenheit his teacher ask him to convert this temperature to degrees celsius what is the boiling point of his substance in degrees celsius
Answers: 3

Chemistry, 23.06.2019 11:20
Try to reduce the amount of leftover ingredients by changing the amount of one, two, or all three starting ingredients. show your stoichiometric calculations below. water 946.36 g sugar 196.86 g lemon juice193.37 g
Answers: 2
You know the right answer?
Achemistry student needs 15.00g of acetic acid for an experiment. he has available 220.g of a 30.2%...
Questions

Mathematics, 03.02.2020 10:50


SAT, 03.02.2020 10:50

Health, 03.02.2020 10:50



Mathematics, 03.02.2020 10:50

Mathematics, 03.02.2020 10:50

English, 03.02.2020 10:50



Social Studies, 03.02.2020 10:50






Mathematics, 03.02.2020 10:51