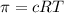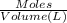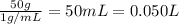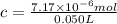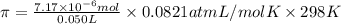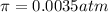Chemistry, 30.07.2019 23:30 makwoods417ow2txa
Lysozyme extracted from chicken egg white has a molar mass of 13,930 g mo1-1. exactly 0.1 g of this protein is dissolved in 50 g of water at 298 k. calculate the vapor pressure lowering, the depression in freezing point, the elevation of boiling point, and the osmotic pressure of this solution. the vapor pressure of pure water at 298 k is 23. 76 mmhg.

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 23:00
Plz choose one of the compounds from the table and explain how you know the numbers of atoms in your formula. is it possible for two different compounds to be made from the exact same two elements? why or why not? with a limited number of elements (less than 120 are known), does this mean we also have a small number of compounds or do we have a large number of compounds in this world?
Answers: 1

Chemistry, 22.06.2019 07:20
The diagrams show objects’ gravitational pull toward each other. which statement describes the relationship between diagram x and y? gravity attracts only larger objects toward one another. gravity attracts larger objects only if they are close to one another. if the masses of the objects increase, then the force between them also increases. if distance between the objects increases, then the amount of force also increases.
Answers: 1

Chemistry, 22.06.2019 10:00
Ahydrogen atom has 1 electron. how many bonds can hydrogen form? a) 1 b) 2 c) 3 d) 4 e) 5
Answers: 3

Chemistry, 22.06.2019 16:00
Answer asap : ( a. how does mucus prevent the entry of pathogens? b. describe two ways white blood cells protect us from pathogens.
Answers: 1
You know the right answer?
Lysozyme extracted from chicken egg white has a molar mass of 13,930 g mo1-1. exactly 0.1 g of this...
Questions

English, 19.09.2019 21:30

Computers and Technology, 19.09.2019 21:30

Mathematics, 19.09.2019 21:30

Social Studies, 19.09.2019 21:30



Advanced Placement (AP), 19.09.2019 21:30

Mathematics, 19.09.2019 21:30



Biology, 19.09.2019 21:30




English, 19.09.2019 21:30


Biology, 19.09.2019 21:30



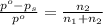
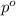 = Vapor Pressure of the pure solvent
= Vapor Pressure of the pure solvent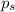 = Vapor Pressure of the solution
= Vapor Pressure of the solution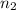 moles of solute
moles of solute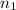 = moles of solvent
= moles of solvent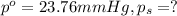
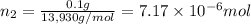
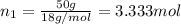
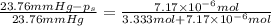
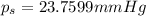
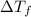 is given by:
is given by: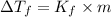
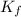 = molal depression constant of solvent
= molal depression constant of solvent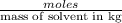
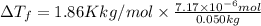
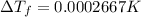
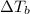 is given by:
is given by: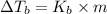
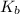 = molal elevation constant of solvent
= molal elevation constant of solvent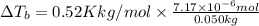
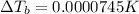
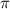 is given as:
is given as: