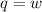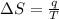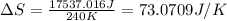Suppose 4.00 mol of an ideal gas undergoes a reversible isothermal expansion from volume v1 to volume v2 = 9v1 at temperature t = 240 k. find (a) the work done by the gas and (b) the entropy change of the gas. (c) if the expansion is reversible and adiabatic instead of isothermal, what is the entropy change of the gas?

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 06:00
If you burn 10 kilograms of wood in a fire (combustion) what is the weight of the products after the fire has finished burning the wood?
Answers: 3

Chemistry, 22.06.2019 11:50
The chemical bond connecting one nucleotide with the next one along the nucleic acid chain is called a
Answers: 3

Chemistry, 22.06.2019 12:00
What is the subscript for oxygen in its molecular formula
Answers: 1

Chemistry, 23.06.2019 00:00
What does an electron configuration for an atom relate to the atoms placement on the periodic table
Answers: 2
You know the right answer?
Suppose 4.00 mol of an ideal gas undergoes a reversible isothermal expansion from volume v1 to volum...
Questions



Chemistry, 02.12.2019 21:31

Health, 02.12.2019 21:31


English, 02.12.2019 21:31

Mathematics, 02.12.2019 21:31

Mathematics, 02.12.2019 21:31

Mathematics, 02.12.2019 21:31




Spanish, 02.12.2019 21:31

Mathematics, 02.12.2019 21:31




English, 02.12.2019 21:31

Mathematics, 02.12.2019 21:31

Biology, 02.12.2019 21:31

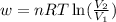
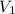 = initial volume of gas =
= initial volume of gas = 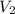 = final volume of gas =
= final volume of gas = 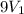
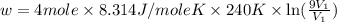
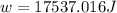
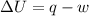
 = internal energy
= internal energy