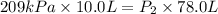Chemistry, 31.07.2019 17:20 houtchhaytang
Acylinder is filled with 10.0l of gas and a piston is put into it. the initial pressure of the gas is measured to be 209.kpa. the piston is now pulled up, expanding the gas, until the gas has a final volume of 78.0l. calculate the final pressure of the gas. be sure your answer has the correct number of significant digits.

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 15:50
The density of glycerin is 1.26grams/centimeter cubed . how many is this? use the conversion rates of and . express your answer to the correct number of significant figures.
Answers: 1

Chemistry, 21.06.2019 20:00
Smog is the term used to describe the combination of fog and smoke
Answers: 1

Chemistry, 22.06.2019 01:30
In a spacecraft, the following reaction occurs: co2(g) + 2lioh(s) -> lico3(s) + h2o(i) (i attached picture of equation) how many liters of carbon dioxide will 4 moles of lithium hydroxide (lioh) absorb? (one mole of any gads occupies 22.4 l under certain conditions of temperature and pressure. assume those conditions for this equation.) 45l 6.0l 3.0l 34l
Answers: 1

Chemistry, 22.06.2019 01:30
When an object falls through the air and encounters air resistance its overall speed will be than if it had not encountered air resistance? (one word answer)
Answers: 2
You know the right answer?
Acylinder is filled with 10.0l of gas and a piston is put into it. the initial pressure of the gas i...
Questions


History, 04.07.2019 07:30

Arts, 04.07.2019 07:30

History, 04.07.2019 07:30


Mathematics, 04.07.2019 07:30



Mathematics, 04.07.2019 07:30

Mathematics, 04.07.2019 07:30




Business, 04.07.2019 07:30

Mathematics, 04.07.2019 07:30



Mathematics, 04.07.2019 07:30

Mathematics, 04.07.2019 07:30

History, 04.07.2019 07:30


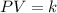

 = initial pressure of the gas = 209 kPa
= initial pressure of the gas = 209 kPa = final pressure of the gas = ?
= final pressure of the gas = ? = initial volume of the gas = 10.0 L
= initial volume of the gas = 10.0 L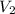 = final volume of the gas = 78.0 L
= final volume of the gas = 78.0 L