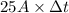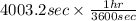Chemistry, 31.07.2019 18:20 chanel2371
Chromium plating can be applied by electrolysis to objects according to the following unbalanced half-reaction: cr2o72- + e- + h+→ cr(s) + h2ohow long (in hours) would it take to apply a chromium plating 0.010 mm thick to a car bumper with a surface area of 0.25 m2 in a cell with a current of 25.0 a? the density of chromium is 7.19 g/cm3.

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 01:00
Which of the following is not a true statement about dwarf planets? a the kuiper belt contains comets, asteroids, and dwarf planets. b ceres is a dwarf planet located in the kuiper belt. c the largest known dwarf planet in the solar system is named eris.
Answers: 2


Chemistry, 22.06.2019 10:30
Which describes fat? a: a carbohydrate that produces energy b: a nucleic acid that directs cell function c: a lipid that stores energy d: a protein that speeds up a chemical reaction
Answers: 1

Chemistry, 22.06.2019 22:30
Vi limitens. vastery test select the correct answer. which statement explains why large atoms are more reactive than small atoms? a. large atoms have valence electrons farther from the nucleus and lose them more readily. b. large atoms have greater ionization energy, which they can utilize during a reaction. c. large atoms have a greater number of electrons that they can lose during a reaction. d. large atoms have more energy levels, so they have more energy to pass on in a reaction. reset next
Answers: 3
You know the right answer?
Chromium plating can be applied by electrolysis to objects according to the following unbalanced hal...
Questions



Physics, 07.07.2019 05:00


Physics, 07.07.2019 05:00

Physics, 07.07.2019 05:00


Mathematics, 07.07.2019 05:00

History, 07.07.2019 05:00






English, 07.07.2019 05:00

Mathematics, 07.07.2019 05:00

Physics, 07.07.2019 05:00

Physics, 07.07.2019 05:00


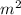 , current (I) = 25 A
, current (I) = 25 A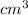
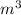 =
= 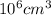
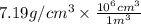
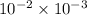 =
= 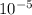 m.
m.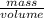
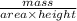
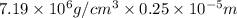
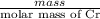
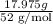
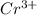 is 579000 C
is 579000 C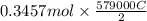 = 100080 C.
= 100080 C. 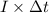
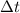 = time in seconds
= time in seconds