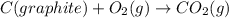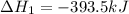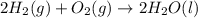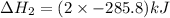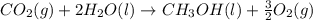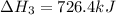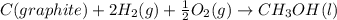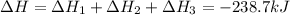Calculate δh for the reaction: c(graphite) + 2h 2(g) + 1/2 o 2(g) => ch 3oh(l) using the following information: c(graphite) + o 2 => co 2(g) δh o = -393.5 kj h 2(g) + 1/2 o 2 => h 2o(l) δh o = -285.8 kj ch 3oh (l) + 3/2 o 2(g) => co 2(g) + 2h 2o(l) δh o = -726.4 kj a. +238.7 kj b. -238.7 kj c. +548.3 kj d. -548.3 kj e. +904.5 kj

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 11:00
Ais a mountain created from eruptions of lava, ash, rocks, and hot gases.
Answers: 1

Chemistry, 22.06.2019 14:00
Will mark brainliest how many electrons can be held in the energy level n = 4?
Answers: 1

Chemistry, 22.06.2019 19:00
Nan element’s square on the periodic table, the number with the greatest numerical value represents the
Answers: 3

Chemistry, 22.06.2019 22:30
You just calculated that the heat of fusion for chloromethane is 6400 j/mol. the heat of fusion for hydrogen is 120 j/mol.? which of the following account for this difference? more than one correcta. chloromethane can absorb more energy at the same temperature. b. hydrogen has stronger intermolecular forces than chloromethane. c. hydrogen molecules can pack more closely than chloromethane molecules. d. chloromethane experiences dipole-dipole interactions. e. chloromethane has a higher molar mass than hydrogen.
Answers: 3
You know the right answer?
Calculate δh for the reaction: c(graphite) + 2h 2(g) + 1/2 o 2(g) => ch 3oh(l) using the follow...
Questions


Mathematics, 15.11.2019 19:31

Mathematics, 15.11.2019 19:31


English, 15.11.2019 19:31


English, 15.11.2019 19:31



Chemistry, 15.11.2019 19:31




History, 15.11.2019 19:31






Computers and Technology, 15.11.2019 19:31

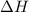 for the given reaction is -238.7 kJ
for the given reaction is -238.7 kJ