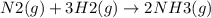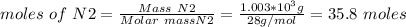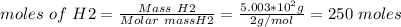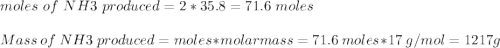Chemistry, 31.07.2019 19:10 zdwilliams1308
Ammonia is produced from the reaction of nitrogen and hydrogen according to the following balanced equation: n2 1 g 2 1 3h2 1 g 2 h 2nh3 1 g 2
a. what is the maximum mass of ammonia that can be produced from a mixture of 1.00 3 103 g n2 and 5.00 3 102 g h2

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 01:30
The table lists pressure and volume values for a particular gas. which is the best estimate for the value of v at p = 7.0 × 103 pascals?
Answers: 3

Chemistry, 22.06.2019 07:00
The organism shown is a free-living one that is anchored to the bottom of ponds and streams during one stage of its life cycle what is the common name for the group to which this organism belong
Answers: 3

Chemistry, 22.06.2019 08:00
Me i dont know what to do! the table compares the number of electrons in two unknown neutral atoms. comparison of electrons atom number of electrons a 10 d 11 use this information to determine the number of valence electrons in the atoms. which of the following correctly compares the stability of the two atoms? both are unreactive. both are highly reactive. a is unreactive and d is reactive. a is reactive and d is unreactive.
Answers: 1

You know the right answer?
Ammonia is produced from the reaction of nitrogen and hydrogen according to the following balanced e...
Questions


Mathematics, 20.04.2021 15:30

Mathematics, 20.04.2021 15:30

Mathematics, 20.04.2021 15:30



Mathematics, 20.04.2021 15:30

Physics, 20.04.2021 15:30



Computers and Technology, 20.04.2021 15:40


English, 20.04.2021 15:40


Physics, 20.04.2021 15:40

English, 20.04.2021 15:40


Social Studies, 20.04.2021 15:40

Mathematics, 20.04.2021 15:40

Chemistry, 20.04.2021 15:40