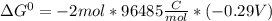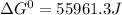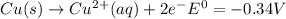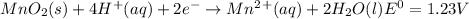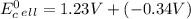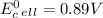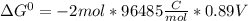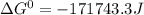Answers: 3


Another question on Chemistry


Chemistry, 22.06.2019 06:10
56.16 gregor mendel was the first scientist to use statistics to analyze scientific data. before mendel's experiments, scientists believed that organisms acquired traits from their environment and passed them on to their offspring. after mendel's discoveries were accepted, scientists realized that traits passed to offspring were the result of genes being passed from parents to offspring. this is an example of pls
Answers: 1

Chemistry, 22.06.2019 12:10
Consider the reaction: n2(g) + o2(g) ⇄ 2no(g) kc = 0.10 at 2000oc starting with initial concentrations of 0.040 mol/l of n2 and 0.040 mol/l of o2, calculate the equilibrium concentration of no in mol/l how would this be done?
Answers: 3

Chemistry, 22.06.2019 17:10
Some liquids can be distilled, but only at temperatures that are so high that it is impractical, or so high the compound decomposes. explain why distillation such compounds at significantly less than atmospheric pressure (some degree of vacuum) would solve this problem.
Answers: 2
You know the right answer?
Use tabulated electrode potentials to calculate ∆g° rxn for each reaction at 25 °c in kj.
(a)...
(a)...
Questions





Mathematics, 27.03.2021 01:50

Mathematics, 27.03.2021 01:50


Mathematics, 27.03.2021 01:50

Mathematics, 27.03.2021 01:50

Mathematics, 27.03.2021 01:50

Mathematics, 27.03.2021 01:50


Mathematics, 27.03.2021 01:50





Mathematics, 27.03.2021 01:50

Mathematics, 27.03.2021 01:50

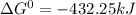 , (b)
, (b) 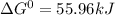 and (c)
and (c) 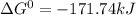
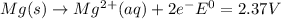
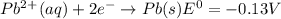
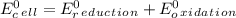
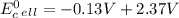
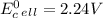
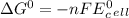
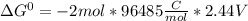
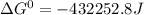
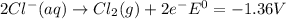
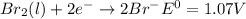
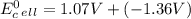
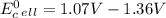
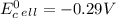
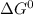 same as we did for part a.
same as we did for part a.