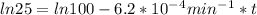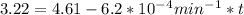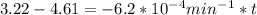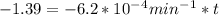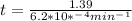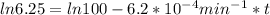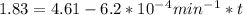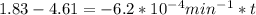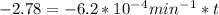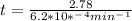Chemistry, 31.07.2019 20:30 YODIIZ6590
If the rate constant for the decomposition of n2o5 is 6.2 × 10−4/min, what is the half-life? (the rate law is first order in n2o5.) how long would it take for the concen- tration of n2o5 to decrease to 25% of its initial value? to 6.25% of its initial value?

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 12:00
What term is applied to a scientist who studies ancient life, including animal and plant fossils a. anthropologist b. dendroclimatologist c. geophysicist d. paleontologist
Answers: 2

Chemistry, 22.06.2019 15:30
A1.5l container holds p.50 grams of an unknown gas at a pressure of 0.44 atm and a temperature of 50.c what is the molar mass of the unknown gas
Answers: 1


Chemistry, 22.06.2019 18:30
Which of the following words describe the reality that the universe looks the same from various perspective
Answers: 3
You know the right answer?
If the rate constant for the decomposition of n2o5 is 6.2 × 10−4/min, what is the half-life? (the r...
Questions

Mathematics, 05.11.2020 07:10







History, 05.11.2020 07:10

Social Studies, 05.11.2020 07:10

Mathematics, 05.11.2020 07:10



Mathematics, 05.11.2020 07:10


Mathematics, 05.11.2020 07:10


Mathematics, 05.11.2020 07:10


Mathematics, 05.11.2020 07:10

Health, 05.11.2020 07:10

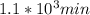 ,
, 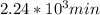 are taken for the concentration to decrease to 25% and
are taken for the concentration to decrease to 25% and 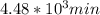 for the concentration to decrease to 6.25% .
for the concentration to decrease to 6.25% . 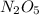 . For first order reaction, rate constant and half life are related to each other as:
. For first order reaction, rate constant and half life are related to each other as: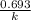
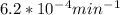
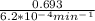
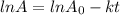
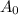 is initial and A is remaining concentration or amounts. k is rate constant and t is the time. Let's plug in the values and do calculations for t.
is initial and A is remaining concentration or amounts. k is rate constant and t is the time. Let's plug in the values and do calculations for t.