
Chemistry, 31.07.2019 20:30 alexandra2442
A12.22 gram sample of an organic compound containing only c, h, and o is analyzed by combustion analysis and 17.91 g co2 and 7.332 g h2o are produced. in a separate experiment, the molar mass is found to be 60.05 g/mol. determine the empirical formula and the molecular formula of the organic compound.

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 13:00
What happens to the average kinetic energy of a gas when the particles of the gas collide against each other at a constant temperature and volume? explain your answer.
Answers: 3


Chemistry, 23.06.2019 05:30
What is the morality of 2.50 l of solution that contains 1.85 mol of anhydrous sodium tetraborate?
Answers: 1

Chemistry, 23.06.2019 09:00
The vapor pressure of water at 25.0°c is 23.8 torr. determine the mass of glucose (molar mass = 180 g/mol) needed to add to 500.0 g of water to change the vapor pressure to 22.8 torr.
Answers: 1
You know the right answer?
A12.22 gram sample of an organic compound containing only c, h, and o is analyzed by combustion anal...
Questions





Geography, 21.08.2019 04:20










English, 21.08.2019 04:30

Chemistry, 21.08.2019 04:30

Mathematics, 21.08.2019 04:30



Mathematics, 21.08.2019 04:30

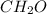 and
and  respectively.
respectively.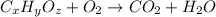
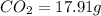
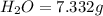
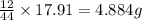 of carbon will be contained.
of carbon will be contained.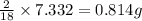 of hydrogen will be contained.
of hydrogen will be contained.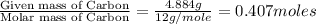


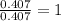
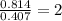

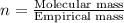
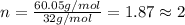
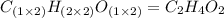
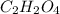 respectively.
respectively.

