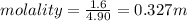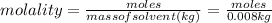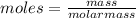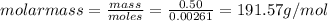Chemistry, 31.07.2019 20:40 linshweyioo5442
Aforensic chemist is given a white powder for analysis. she dissolves 0.50 g of the substance in 8.0 g of benzene. the solution freezes at 3.9°c. can the chemist conclude that the compound is cocaine (c17h21n04)? what assumptions are made in the analysis? the freezing point of benzene is 5.5°c.

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 11:10
Which of the following shapes would represent a molecule with two bonded atoms and 3 lone pairs on only one of them , trigonal planar , bent , trigonal pyramidal , linear
Answers: 1

Chemistry, 22.06.2019 15:20
Water is initially present in a state where its molecules are far apart. during a change of state, its molecules slow down. which change of state has most likely taken place? from a gas to a liquid from a liquid to a gas from a solid to a liquid from a gas to a plasma
Answers: 1

Chemistry, 22.06.2019 21:30
In science class richard learns that a substance has a boiling point of 230 fahrenheit his teacher ask him to convert this temperature to degrees celsius what is the boiling point of his substance in degrees celsius
Answers: 3

Chemistry, 22.06.2019 21:30
Athe top of a hill, an athlete on a skateboard has x joules of mechanical energy. how much mechanical energy will she have at the bottom of the hill? ignore the effects of friction.
Answers: 1
You know the right answer?
Aforensic chemist is given a white powder for analysis. she dissolves 0.50 g of the substance in 8.0...
Questions

Mathematics, 24.02.2020 09:50



Mathematics, 24.02.2020 09:53

Mathematics, 24.02.2020 09:54


Mathematics, 24.02.2020 09:56

Business, 24.02.2020 09:59

Physics, 24.02.2020 09:59

Social Studies, 24.02.2020 09:59



History, 24.02.2020 10:01


Mathematics, 24.02.2020 10:04

English, 24.02.2020 10:04