
Chemistry, 31.07.2019 21:20 smelcher3900
Hydrogen peroxide decomposes into water and oxygen in a first-order process. h2o2(aq) --> h2o(l) + 1/2 o2(g)at 20.0 °c, the half-life for the reaction is 3.92 x 104 seconds. if the initial concentration of hydrogen peroxide is 0.52 m, what is the concentration after 7.00 days? 1.2 x 10-5 m0.034 m0.074 m0.22 m0.52 m

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 00:10
According to the diagram; a) identify the anode of the cell and write the half-reaction that occurs there b) write the overall equation for the reaction that occurs as the cell operates c) calculate the value of the standard cell potential ,e cell. d)write the shorthand notation of the cell above e)indicate the flow of the electrons on the diagram
Answers: 3

Chemistry, 22.06.2019 04:00
Tin has ten stable isotopes. the heaviest, 124sn, makes up 5.80% of naturally occuring tin atoms. how many atoms of 124sn are present in 82.0 g of naturally occurring tin? what is the total mass of the 124sn atoms in this sample?
Answers: 3

Chemistry, 22.06.2019 05:30
What type of reaction is shown below? check all that apply. 2h2o2 → 2h2o + o2 synthesis decomposition combustion
Answers: 3

You know the right answer?
Hydrogen peroxide decomposes into water and oxygen in a first-order process. h2o2(aq) --> h2o(l)...
Questions






Health, 10.01.2020 22:31

Computers and Technology, 10.01.2020 22:31


English, 10.01.2020 22:31

English, 10.01.2020 22:31



Mathematics, 10.01.2020 22:31


English, 10.01.2020 22:31

English, 10.01.2020 22:31

Physics, 10.01.2020 22:31



Mathematics, 10.01.2020 22:31

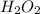 after 7.00 days is
after 7.00 days is 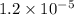 M
M![[H_{2}O_{2}]=[H_{2}O_{2}]_{0}\times (0.5)^{(\frac{t}{t_{0.5}})}](/tpl/images/0155/7555/57b7f.png)
![[H_{2}O_{2}]_{0}](/tpl/images/0155/7555/0e2b6.png) is initial concentration of
is initial concentration of 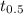 is half-life
is half-life![[H_{2}O_{2}]= 0.52\times (0.5)^{\frac{604800}{39200}}](/tpl/images/0155/7555/78886.png)
![[H_{2}O_{2}]](/tpl/images/0155/7555/955b6.png) =
= 

