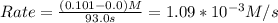Chemistry, 31.07.2019 21:30 navjitdosanjh20
For the gas phase decomposition of phosphine at 120 °c, the rate of the reaction is determined by measuring the appearance of h2. 4 ph3(g)p4(g) + 6 h2(g) at the beginning of the reaction, the concentration of h2 is 0 m. after 93.0 s the concentration has increased to 0.101 m. what is the rate of the reaction? (mol h2/l) /s

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 02:00
Which of the following is not a good technique for managing used oil? a) have specific, labeled catch pans available for technicians who are collecting oil b) spills in your shop and any releases on pavement or outside should be poured down a drain c) do not use oil containers for antifreeze or other non-similar fluids d) be prepared for oil spills with the proper absorbents
Answers: 1

Chemistry, 22.06.2019 03:50
What is the temperature of one mole of helium gas at stp?
Answers: 3

Chemistry, 22.06.2019 13:00
Asubstance is a good conductor of electricity which of the following best explains a probable position of the substance in a periodic table
Answers: 3

Chemistry, 23.06.2019 07:00
Write a hypothesis that answers the lesson question, “while observing a chemical reaction, how can you tell which reactant is limiting? ” hypothesis: if a substance is the limiting reactant, then . . because . .
Answers: 1
You know the right answer?
For the gas phase decomposition of phosphine at 120 °c, the rate of the reaction is determined by me...
Questions


Mathematics, 31.01.2020 08:44

Mathematics, 31.01.2020 08:44


Mathematics, 31.01.2020 08:44

History, 31.01.2020 08:44





Biology, 31.01.2020 08:44

Mathematics, 31.01.2020 08:44

Biology, 31.01.2020 08:44



Spanish, 31.01.2020 08:44


Geography, 31.01.2020 08:45


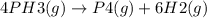
![Rate = +\frac{1}{6}*\frac{\Delta [H2]]}{\Delta t}](/tpl/images/0155/7784/3af01.png)
![Rate = +\frac{1}{6}*\frac{C2[H2]-C1[H2]}{\Delta t}](/tpl/images/0155/7784/69ced.png)