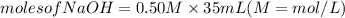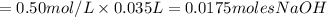Chemistry, 31.07.2019 22:10 richdakid26
If it requires 35.0 milliliters of 0.50 molar naoh to neutralize 25.0 milliliters of hcl, what is the concentration of the hcl solution? (3 points)
balanced equation: naoh + hcl yields nacl + h2o
select one:
a. 0.36 m hcl
b. 0.70 m hcl
c. 1.1 m hcl
d. 1.4 m hcl

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 17:40
Which statement about hf is true? it is zero for any compound in its standard state. it is positive when the bonds of the product store more energy than those of the reactants. it is negative when a compound forms from elements in their standard states. it is zero for any element that is in the liquid state.
Answers: 1

Chemistry, 23.06.2019 03:30
How many grams of sodium chloride are in 250ml of a 2.5m naci solution
Answers: 1

Chemistry, 23.06.2019 12:30
D5w is shorthand for a 5% glucose rehydration fluid used in ivs. the doctor orders 1200 ml d5w@ 30 gtts/min. you have an iv tube that delivers 18 gtts/cc. how many hours will it take for the 1200 cc bottle to infuse?
Answers: 2

Chemistry, 23.06.2019 16:00
The table below shows a comparison of the different gas laws. some cells have been left blank. name variables constants equation boyle's law pressure, volume ? pv = k charles’s law volume, temperature ? v = kt ? temperature, pressure volume, moles of gas p = kt ? pressure, temperature, volume ? which are assumed to be constant while using the combined gas law? 1. pressure 2. number of moles 3. volume and moles of gas 4. pressure and temperature
Answers: 1
You know the right answer?
If it requires 35.0 milliliters of 0.50 molar naoh to neutralize 25.0 milliliters of hcl, what is th...
Questions

Mathematics, 21.06.2019 12:40

Mathematics, 21.06.2019 12:40



History, 21.06.2019 12:40


Mathematics, 21.06.2019 12:40

Mathematics, 21.06.2019 12:40

Chemistry, 21.06.2019 12:40

Social Studies, 21.06.2019 12:40



History, 21.06.2019 12:40

Mathematics, 21.06.2019 12:40

History, 21.06.2019 12:40

Mathematics, 21.06.2019 12:40

Mathematics, 21.06.2019 12:40

Mathematics, 21.06.2019 12:40

Biology, 21.06.2019 12:40


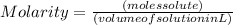 and its unit is mol/L
and its unit is mol/L