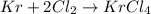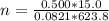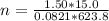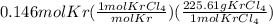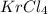Chemistry, 31.07.2019 22:10 lapointayyy6388
A15.0-l rigid container was charged with 0.500 atm of kryp‑ ton gas and 1.50 atm of chlorine gas at 350.8c. the krypton and chlorine react to form krypton tetrachloride. what mass of krypton tetrachloride can be produced assuming 100% yield?

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 14:00
Anthracite is so hard and pure it is also referred to as a renewable resource metamorphic rock hot bituminous coal dirty fuel
Answers: 1

Chemistry, 23.06.2019 00:30
What is calcium oxide+diphosphorus pentoxide--> calcium phosphate balanced
Answers: 1


Chemistry, 23.06.2019 08:30
Imagine you are a business executive who wants to pursue an environment policy for your company that limits pollution and uses fewer raw materials but would cost more what might be the discussion to your next broad meeting how would you make your case to your shareholders
Answers: 1
You know the right answer?
A15.0-l rigid container was charged with 0.500 atm of kryp‑ ton gas and 1.50 atm of chlorine gas at...
Questions

Mathematics, 25.07.2019 19:00

Arts, 25.07.2019 19:00





Biology, 25.07.2019 19:00

Mathematics, 25.07.2019 19:00

Mathematics, 25.07.2019 19:00





Mathematics, 25.07.2019 19:00

Mathematics, 25.07.2019 19:00



History, 25.07.2019 19:00


Social Studies, 25.07.2019 19:00