
Chemistry, 31.07.2019 23:10 aubriebv2020
The solubility of cd(oh)2 can be increased through formation of the complex ion cdbr2−4 (kf=5×103). if solid cd(oh)2 is added to a nabr solution, what would the initial concentration of nabr need to be in order to increase the molar solubility of cd(oh)2 to 1.0×10−3 moles per liter?

Answers: 2


Another question on Chemistry


Chemistry, 22.06.2019 20:10
The lattice enthalpy (formation of ionic solid from ions in the gas phase) for agcl(s) is -916 kj/mol and the hydration enthalpy (dissolution of gaseous ions into water) is -850 kj/mol. how much heat (in joules) is involved in forming 1l of saturated agcl solution (1.8 × 10-4 g / 100 ml water) by dissolving agcl(s)? assume solution volume does not change much upon dissolution. the equations are given below. ag+(g) + cl−(g) æ agcl(s)
Answers: 3


Chemistry, 22.06.2019 21:30
While in europe, if you drive 125 km per day, how much money would you spend on gas in one week if gas costs 1.10 euros per liter and your car’s gas mileage is 32.0 mi/gal? assume that 1 euro=1.26 dollars
Answers: 2
You know the right answer?
The solubility of cd(oh)2 can be increased through formation of the complex ion cdbr2−4 (kf=5×103)....
Questions


Mathematics, 02.04.2020 12:05


Mathematics, 02.04.2020 12:05

Mathematics, 02.04.2020 12:05

Mathematics, 02.04.2020 12:06

Mathematics, 02.04.2020 12:06




Mathematics, 02.04.2020 12:16

Mathematics, 02.04.2020 12:16

Mathematics, 02.04.2020 12:26


Mathematics, 02.04.2020 12:26



Mathematics, 02.04.2020 12:26

Mathematics, 02.04.2020 12:26

Mathematics, 02.04.2020 12:26

![Cd^{2+} + 4Br^{-}\rightleftharpoons [CdBr_{4}]^{2-} .....Kf =5*10^{3}](/tpl/images/0156/0621/b20e2.png) -----(1)
-----(1)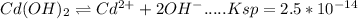 ---(2)
---(2)![Cd(OH)_{2} + 4Br^{-}\rightleftharpoons [CdBr_{4}]^{2-}+2OH^{-}](/tpl/images/0156/0621/e6b8f.png)
![K = K_{f}*K_{sp} = 5*10^{3}*2.5*10^{-14}=\frac{[CdBr_{4}^{2-}][OH^{-}]^{2}}{[Br{-}]^{4}}](/tpl/images/0156/0621/29c0f.png)
![12.5*10^{-11} =\frac{1*10^{-3} *[2*10^{-3}]^{2}}{[Br-]^{4} }\\](/tpl/images/0156/0621/ca71c.png)
![[Br-] = 2.38 M](/tpl/images/0156/0621/628e8.png)


