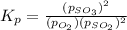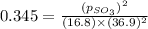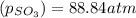Chemistry, 31.07.2019 23:10 yournerdybirdyp43oi3
At 900.0 k, the equilibrium constant (kp) for the following reaction is 0.345. 2so2 + o2 (g) →2 so3 (g) at equilibrium, the partial pressure of so2 is 36.9 atm and that of o2 is 16.8 atm. the partial pressure of so3 is atm.

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 02:30
Asa choose the correct set of reaction coefficients to properly balance the following chemical equation according to the law of conservation of mass: __s8 + __o2 ==> __so2 1, 1, 8 1, 8, 1 1, 8, 8 8, 1, 1
Answers: 1

Chemistry, 22.06.2019 17:30
Upon decomposition, one sample of magnesium fluoride produced 1.65 kg of magnesium and 2.56 kg of fluorine. a second sample produced 1.32 kg of magnesium. part a how much fluorine (in grams) did the second sample produce?
Answers: 2

Chemistry, 22.06.2019 18:10
Given the following at 25c calculate delta hf for hcn (g) at 25c. 2nh3 (g) +3o2 (g) + 2ch4 (g) > 2hcn (g) + 6h2o (g) delta h rxn= -870.8 kj. delta hf=-80.3 kj/mol for nh3 (g), -74.6 kj/mol for ch4, and -241.8 kj/mol for h2o (g)
Answers: 1

Chemistry, 22.06.2019 20:30
Citric acid has a ph between 1 and 3. it is considered to be aa. weak acidb. weak basec. strong based. strong acid
Answers: 2
You know the right answer?
At 900.0 k, the equilibrium constant (kp) for the following reaction is 0.345. 2so2 + o2 (g) →2 so3...
Questions


Mathematics, 21.07.2020 21:01






Mathematics, 21.07.2020 21:01

Mathematics, 21.07.2020 21:01

Mathematics, 21.07.2020 21:01



Mathematics, 21.07.2020 21:01

Mathematics, 21.07.2020 21:01



History, 21.07.2020 21:01

Mathematics, 21.07.2020 21:01

Chemistry, 21.07.2020 21:01

Mathematics, 21.07.2020 21:01

 is, 88.84 atm
is, 88.84 atm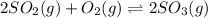
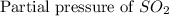 = 36.9 atm
= 36.9 atm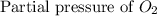 = 16.8 atm
= 16.8 atm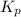 will be,
will be,