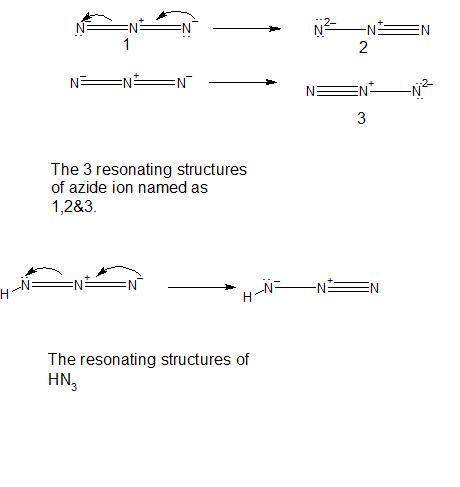
Be sure to answer all parts. pure hn3 (atom sequence hnnn) is explosive. in aqueous solution, it is a weak acid that yields the azide ion, n3−. draw one resonance structure for n3− and one resonance structure for hn3. include all lone pair electrons and nonzero formal charges in your structures.

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 22:30
Naoki's bicycle has a mass of 10 kg. if naoki sits on her bicycle and starts pedaling with a force of 168 n, causing an acceleration of 2.8 m/s2, what is naoki's mass?
Answers: 1

Chemistry, 22.06.2019 06:40
Ted and emily played a mixed doubles tennis match against jack and brenda. in the second match. ted and brenda played against jack and emily. which type of chemical reaction does the situation demonstrate?
Answers: 3

Chemistry, 22.06.2019 12:00
A5.000 g sample of niso4 h2o decomposed to give 2.755 g of anhydrous niso4. what is the formula of the hydrate? what is the full chemical name for the hydrate? what is the molar mass of the hydrate? niso4•_h2o what is the mass % of water in the hydrate?
Answers: 1

Chemistry, 22.06.2019 13:50
Read the chemical equation. 2c2h2 + 5o2 → 4co2 + 2h2o which of the following statements would be correct if one mole of c2h2 was used in this reaction? one mole of oxygen was used in this reaction. five moles of oxygen were used in this reaction. four moles of carbon dioxide were produced from this reaction. two moles of carbon dioxide were produced from this reaction.
Answers: 3
You know the right answer?
Be sure to answer all parts. pure hn3 (atom sequence hnnn) is explosive. in aqueous solution, it is...
Questions


Mathematics, 20.09.2020 03:01


English, 20.09.2020 03:01

Computers and Technology, 20.09.2020 03:01

Mathematics, 20.09.2020 03:01


Biology, 20.09.2020 03:01

Biology, 20.09.2020 03:01

Mathematics, 20.09.2020 03:01

Business, 20.09.2020 03:01

English, 20.09.2020 03:01

Mathematics, 20.09.2020 03:01







History, 20.09.2020 03:01