
Chemistry, 01.08.2019 00:20 bcozort611
For the reaction co(g) + 3 h2(g) ⇌ h2o(g) + ch4(g), kc = 189 at 1000 k. if a vessel is filled with these gases such that the initial concentrations are [co] = 0.0360 m, [h2] = 0.0450 m, [h2o] = 0.0200 m, and [ch4] = 0.0310 m, in which direction will a reaction occur and why?

Answers: 2


Another question on Chemistry

Chemistry, 23.06.2019 05:40
Why is any chemical reaction always balanced? give reasons and explain the easiest way to solve the balancing problems in chemical equations with stoichiometric coefficients upto 20 as hit and trial doesn't always work. give full reasoning
Answers: 1

Chemistry, 23.06.2019 13:00
How long could you survive without electricity? what parts of your life would be affected by loss of electricity? should you prepare for an electricity outage, and if so, how would you prepare? what backup system could your family or community install to generate limited amounts of electricity during an outage? how does this system create an electric force field and generate electric current?
Answers: 2

Chemistry, 23.06.2019 14:00
How many moles of oxygens atoms are present in 5.00 mol of diphosphorus of fe2(so4)3
Answers: 2

Chemistry, 23.06.2019 15:50
Many radioactive atoms that have large masses undergo radioactive decay by releasing a particle that is identical to a helium-4 nucleus. what changes in the original atom are expected as a result of this natural phenomenon? the atomic number and the mass number will decrease. the atomic number and the mass number will increase. the atomic number will increase, and the mass number will decrease. the atomic number will decrease, and the mass number will increase.
Answers: 2
You know the right answer?
For the reaction co(g) + 3 h2(g) ⇌ h2o(g) + ch4(g), kc = 189 at 1000 k. if a vessel is filled with t...
Questions



Mathematics, 10.02.2021 23:20

Mathematics, 10.02.2021 23:20




Mathematics, 10.02.2021 23:20

Mathematics, 10.02.2021 23:20

Biology, 10.02.2021 23:20

Mathematics, 10.02.2021 23:20


English, 10.02.2021 23:20


Advanced Placement (AP), 10.02.2021 23:20




Chemistry, 10.02.2021 23:20

Mathematics, 10.02.2021 23:20

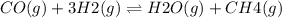
![Qc = \frac{[Products]}{[Reactants} \\\\For\ the\ given\ reaction\\\\Qc = \frac{[H2O][CH4]}{[CO][H2]^{3} } -----(1)](/tpl/images/0156/2334/36d57.png)
![Qc= \frac{[0.0200][0.0310]}{[0.0360][0.0450]^{3} } =189](/tpl/images/0156/2334/3395f.png)


