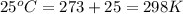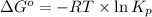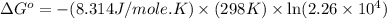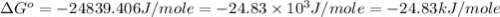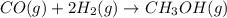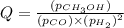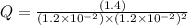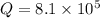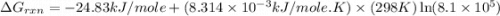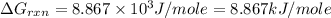Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 06:30
Design techniques and materials that reduce the negative environmental impact of a structure are referred to as
Answers: 2

Chemistry, 22.06.2019 16:00
Uranium can supply energy for the worlds electricity without admitting harmful greenhouse gases which of these statements best describes an outcome of uranium mining
Answers: 1

Chemistry, 22.06.2019 19:30
Phosphorous can form an ion called phosphide, which has the formula p3−. this ion can form an ion called phosphide, which has the formula p3−. this ion properties very similar to those of pforms when a phosphorus atom loses three protonsis called a cationcontains 18 electrons
Answers: 2

Chemistry, 22.06.2019 22:00
Plz ill give u brainliest which of the following steps is not likely to take place during cellular respiration? a.oxygen combines with carbon of simple sugar. b. energy molecule transfers energy to cells. d. energy is used up.
Answers: 3
You know the right answer?
Consider the following reaction: co(g)+2h2(g)⇌ch3oh(g) kp=2.26×104 at 25 ∘c. calculate δgrxn for th...
Questions






Biology, 03.09.2020 21:01






Health, 03.09.2020 21:01

Mathematics, 03.09.2020 21:01

Business, 03.09.2020 21:01


Mathematics, 03.09.2020 21:01




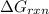 is,
is, 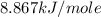
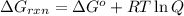 ............(1)
............(1)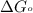 = standard Gibbs free energy
= standard Gibbs free energy