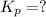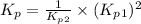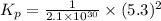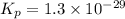Chemistry, 01.08.2019 01:20 christophergaudette0
Consider the following reactions and their respective equilibrium constants: no(g)+12br2(g)⇌nobr(g)kp=5.3 2no(g)⇌n2(g)+o2(g)kp=2.1×1030 use these reactions and their equilibrium constants to predict the equilibrium constant for the following reaction: n2(g)+o2(g)+br2(g)⇌2nobr(g)

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 07:00
What effect does a decrease in temperature have on the overall rate of a chemical reaction? a decrease in temperature decreases . the reaction rate will
Answers: 1

Chemistry, 22.06.2019 14:00
Ascientist measures the speed of sound in a monatomic gas to be 449 m/s at 20∘c. what is the molar mass of this gas?
Answers: 2


Chemistry, 22.06.2019 18:00
An object displaces 652 ml of water. the volume of the object is: 0.652 cm³ 6.52 cm³ 65.2 cm³ 652 cm³
Answers: 3
You know the right answer?
Consider the following reactions and their respective equilibrium constants: no(g)+12br2(g)⇌nobr(g)...
Questions



Mathematics, 31.12.2019 00:31

History, 31.12.2019 00:31



Biology, 31.12.2019 00:31



History, 31.12.2019 00:31


Mathematics, 31.12.2019 00:31





History, 31.12.2019 00:31



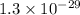
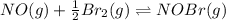 ;
; 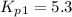
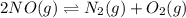 ;
; 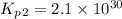
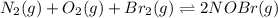 ;
;