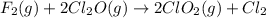Chemistry, 01.08.2019 01:30 unknown9263
The following initial rate data apply to the reaction f2(g) + 2cl2o(g) ® 2clo2(g) + cl2(g).which of the following is the rate law (rate equation) for this reaction? rate = k[f2][cl2o]rate = k[f2]2[cl2o]2rate = k[f2][cl2o]2rate = k[f2]2[cl2o]4rate = k[f2]2[cl2o]

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 06:30
This drawing shows a human body system. what is the primary function of this body system?
Answers: 3

Chemistry, 22.06.2019 12:00
Explain what happens at the saturation point when adding salt to water at room temperature.
Answers: 1


Chemistry, 23.06.2019 01:00
Chromium(iii) sulfate is a transition metal compound containing the metal chromium and the polyatomic ion sulfate. the oxidation state of chromium in this compound is , and the chemical formula of the compound is ( ) . reset next
Answers: 3
You know the right answer?
The following initial rate data apply to the reaction f2(g) + 2cl2o(g) ® 2clo2(g) + cl2(g).which of...
Questions



Mathematics, 11.03.2021 04:20


Social Studies, 11.03.2021 04:20



English, 11.03.2021 04:20

Mathematics, 11.03.2021 04:20

Mathematics, 11.03.2021 04:20

Chemistry, 11.03.2021 04:20






Mathematics, 11.03.2021 04:20



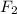 ]
] ![[Cl_{2}O]^{2}](/tpl/images/0156/4052/463f7.png)