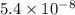Chemistry, 01.08.2019 02:20 kdenormandie3122
In air, at 25 ºc and 1.00 atm, the concentrations of n2 and o2 are 0.033 m and 0.00180, respectively. the reaction n2(g) + o2 (g) 2 no (g) has kc= 4.8 x 10-11 at 25 °c taking the given concentrations as the initial concentrations, calculate the equilibrium concentration of no at 25 °c

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 22:30
Determine the wavelength of the light absorbed when an electron in a hydrogen atom makes a transition from an orbital in the n=3 level to an orbital in the n=7 level.
Answers: 2

Chemistry, 22.06.2019 18:20
Categorize them by metal, nonmetal, in periodic tableductilenon-ductilemalleableoften gain electrons easilygood conductorpoor conductorcan be liquidselements
Answers: 2

Chemistry, 22.06.2019 19:40
What is the wavelength of a 3*10^12 hz infrared wave a 3*10^20m b 1* 10^4m c 3*10^-3m d 1*10^-4 m
Answers: 1

Chemistry, 23.06.2019 03:50
Show how to convert the temperature 84.7° c to kelvin. include all steps and label the final answer.
Answers: 1
You know the right answer?
In air, at 25 ºc and 1.00 atm, the concentrations of n2 and o2 are 0.033 m and 0.00180, respectively...
Questions

Chemistry, 05.07.2019 00:00


Chemistry, 05.07.2019 00:00

Mathematics, 05.07.2019 00:00

History, 05.07.2019 00:00

Biology, 05.07.2019 00:00

History, 05.07.2019 00:00




Chemistry, 05.07.2019 00:00


History, 05.07.2019 00:00

Mathematics, 05.07.2019 00:00

History, 05.07.2019 00:00

Mathematics, 05.07.2019 00:00




Mathematics, 05.07.2019 00:00

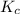 is
is 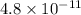
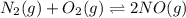
![K_{c} = \frac{[NO]^{2}}{[N_{2}][O_{2}]}](/tpl/images/0156/5544/a7a07.png)
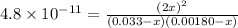
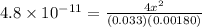
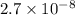 M
M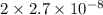 M =
M =