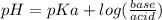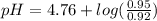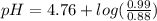Calculate the ph of 1.00 l of the buffer 0.95 m ch3coona/0.92 m ch3cooh before and after the addition of the following species. (assume there is no change in volume.) (a) ph of starting buffer: (b) ph after addition of 0.040 mol naoh: (c) ph after further addition of 0.100 mol hcl:

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 22:00
If the particles in a sample of matter have an orderly arrangement and move only in place, the sample is a
Answers: 1

Chemistry, 22.06.2019 03:30
What is the relationship of air masses and the temperature of oceans?
Answers: 1

Chemistry, 22.06.2019 03:40
In an effort to address concerns about global warming, a power plant in portland,oregon is designed to take all of its exhaust gases from its boilers and recycle the co2 using the solvay process to make sodium hydrogen carbonate. the reaction is shown below. nh3(g) + h2o(l) + co2(g) + nacl(aq) → nahco3(aq) + nh4cl(aq) how many liters each of nh3 and co2 (both at stp) would be consumed to produce 3.00 kg of sodium bicarbonate? the volume of both nh3 and co2 would be
Answers: 1

Chemistry, 22.06.2019 17:00
In a heat engine of 1000 j of heat enters the system and the piston does 500 j of work what is the final internal energy of the system if the inital energy was 2000 j we have to do all of these down here 1)write the equation 2)list out your know variables 3)plug the numbers into the equations 4)solve 5)write your solution statemtn that includes inital energuy and final energuy added
Answers: 1
You know the right answer?
Calculate the ph of 1.00 l of the buffer 0.95 m ch3coona/0.92 m ch3cooh before and after the additio...
Questions

Mathematics, 23.06.2019 20:30



Mathematics, 23.06.2019 20:30


Mathematics, 23.06.2019 20:30



English, 23.06.2019 20:30






Mathematics, 23.06.2019 20:30


Chemistry, 23.06.2019 20:30



Mathematics, 23.06.2019 20:30