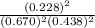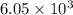Chemistry, 01.08.2019 03:20 makeithappen60
At 250°c an equilibrium mixture of sbcl3(g), cl2(g), and sbcl5(g) has the partial pressures 0.670 bar, 0.438 bar, and 0.228 bar, respectively. calculate the new equilibrium pressures if the volume of the reaction vessel is doubled.

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 14:30
Calculate the mass of carbon in 97.0 g of sucrose c12h22o11
Answers: 3

Chemistry, 22.06.2019 15:30
The reactions of photosynthesis occur in the of plant cell? a.mitochondria. b. lysosomes. c. chloroplasts. d. chlorophyll
Answers: 1

Chemistry, 22.06.2019 17:10
Increasing the substrate concentration in an enzymatic reaction could overcome which of the following? a) the need for a coenzymeb) allosteric inhibitionc) competitive inhibitiond) insufficient cofactors
Answers: 1

Chemistry, 22.06.2019 21:30
Liquid ammonia is produced at high temperatures and under great pressure in a tank by passing a mixture of nitrogen gas and hydrogen gas over an iron catalyst. the reaction is represented by the following equation. n2(g) + 3h2(g) → 2nh3(g) changing all but one experimental condition will affect the amount of ammonia produced. that condition is a) increasing the concentration of both reactants b) changing the temperature within the tank c) decreasing the pressure within the tank. d) increasing only the amount of nitrogen present.
Answers: 1
You know the right answer?
At 250°c an equilibrium mixture of sbcl3(g), cl2(g), and sbcl5(g) has the partial pressures 0.670 ba...
Questions


Computers and Technology, 03.12.2020 02:10

History, 03.12.2020 02:10

Mathematics, 03.12.2020 02:10

Mathematics, 03.12.2020 02:10


History, 03.12.2020 02:10


Computers and Technology, 03.12.2020 02:10


Mathematics, 03.12.2020 02:10

Mathematics, 03.12.2020 02:10

History, 03.12.2020 02:10






Physics, 03.12.2020 02:10

Mathematics, 03.12.2020 02:10

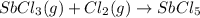
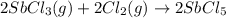
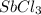 is 0.670 bar,
is 0.670 bar, 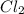 is 0.438 bar and
is 0.438 bar and 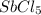 is 0.228 bar.
is 0.228 bar. ![K_{p} = \frac{[P_{SbCl_{5}}]^{2}}{[P_{SbCl_{3}}]^{2}[P_{Cl_{2}}]^{2}}](/tpl/images/0156/7126/bcfbb.png)