
Chemistry, 01.08.2019 03:20 malachilaurenc
The potential energy diagram for a reaction starts at 180 kj and ends at 300 kj. what type of reaction does the diagram best represent?
exothermic reaction
endothermic reaction
reaction between two solids
reaction between two liquids

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 04:50
Write the overall equation for the reaction for lithium battery
Answers: 2

Chemistry, 22.06.2019 14:50
The table compares the number of electrons in two unknown neutral atoms. comparison of electrons atom number of electrons a 9 d 11 use this information to determine the number of valence electrons in the atoms. which of the following correctly compares the stability of tthe table compares the number of electrons in two unknown neutral atoms. comparison of electrons atom number of electrons a 9 d 11 use this information to determine the number of valence electrons in the atoms. which of the following correctly compares the stability of the two atoms? both are unreactive. both are highly reactive. a is unreactive and d is reactive. a is reactive and d is unreactive.
Answers: 3

Chemistry, 22.06.2019 19:00
How many moles of cu are needed to react with 5.8 moles of agno3? cu + 2 agno3 → cu(no3)2 + 2 ag
Answers: 3

Chemistry, 23.06.2019 07:30
Assignment directions: pick one of the following chemists and perform a bit of research on him/her. answer the following questions. alice hamilton rosalind franklin marie curie gertrude b. elion ada yonath henry cavendish robert boyle antoine lavoisier mario j. molina svante arrhenius
Answers: 1
You know the right answer?
The potential energy diagram for a reaction starts at 180 kj and ends at 300 kj. what type of reacti...
Questions





History, 19.03.2020 08:57

English, 19.03.2020 08:57

History, 19.03.2020 08:57


History, 19.03.2020 08:57

English, 19.03.2020 08:57

Biology, 19.03.2020 08:57



Physics, 19.03.2020 08:57

Mathematics, 19.03.2020 08:57





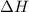 for the reaction comes out to be negative.
for the reaction comes out to be negative.


