
Chemistry, 01.08.2019 03:20 lovelyheart5337
1. in an equilibrium experiment, acetic acid (which is a weak acid) is mixed with sodium acetate (a soluble salt), with methyl orange as an indicator. explain this phenomenon by using the common ion effect. include equations in your explanation.

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 23:50
How will the emission of an alpha particle affect the atomic number of an atom
Answers: 3

Chemistry, 22.06.2019 06:00
In 1901, thomas edison invented the nickel-iron battery. the following reaction takes place in the battery. fe(s) + 2 nio(oh)(s) + 2 h2o(l) fe(oh)2(s) + 2 ni(oh)2(aq) how many mole of fe(oh)2, is produced when 5.35 mol fe and 7.65 mol nio(oh) react?
Answers: 3

Chemistry, 22.06.2019 20:40
What effect would average population growth have on land usage? a. urban use of land would rise to more than 30 percent of available land. b. industrial use of land would rise to more than 30 percent of available land. c. the percentage of available land used as cropland would stay the same. d. cropland would fall to about 10 percent of available land.
Answers: 1

Chemistry, 22.06.2019 21:30
In science class richard learns that a substance has a boiling point of 230 fahrenheit his teacher ask him to convert this temperature to degrees celsius what is the boiling point of his substance in degrees celsius
Answers: 3
You know the right answer?
1. in an equilibrium experiment, acetic acid (which is a weak acid) is mixed with sodium acetate (a...
Questions



Mathematics, 18.12.2020 17:40

Biology, 18.12.2020 17:40

Arts, 18.12.2020 17:40

Mathematics, 18.12.2020 17:40


English, 18.12.2020 17:40

English, 18.12.2020 17:40




Business, 18.12.2020 17:40




History, 18.12.2020 17:40

Mathematics, 18.12.2020 17:40

History, 18.12.2020 17:40

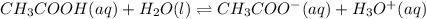
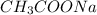 ) is a salt of weak acid, that is,
) is a salt of weak acid, that is, 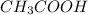 and strong base, that is, NaOH. Therefore, aqueous solution of sodium acetate will be basic in nature.
and strong base, that is, NaOH. Therefore, aqueous solution of sodium acetate will be basic in nature.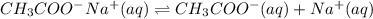
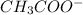 ion.
ion.

