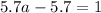Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 15:00
‘which reaction would most likely require the use of an inert electrode?
Answers: 1

Chemistry, 22.06.2019 22:30
Vi limitens. vastery test select the correct answer. which statement explains why large atoms are more reactive than small atoms? a. large atoms have valence electrons farther from the nucleus and lose them more readily. b. large atoms have greater ionization energy, which they can utilize during a reaction. c. large atoms have a greater number of electrons that they can lose during a reaction. d. large atoms have more energy levels, so they have more energy to pass on in a reaction. reset next
Answers: 3

Chemistry, 22.06.2019 23:30
What are the similarities between compounds and mixtures?
Answers: 3

Chemistry, 23.06.2019 04:20
The graph shows one consequence of urban sprawl. how did urban sprawl contribute to the change in biodiversity
Answers: 2
You know the right answer?
For the chemical equation so2(g)+no2(g)↽−−⇀so3(g)+no(g) the equilibrium constant at a certain temper...
Questions

Mathematics, 13.08.2020 01:01

Mathematics, 13.08.2020 01:01

Mathematics, 13.08.2020 01:01

Mathematics, 13.08.2020 01:01



English, 13.08.2020 01:01

Mathematics, 13.08.2020 01:01

Mathematics, 13.08.2020 01:01



Mathematics, 13.08.2020 01:01




Mathematics, 13.08.2020 01:01


Mathematics, 13.08.2020 01:01

Health, 13.08.2020 01:01

![Kc=\frac{[NO][SO_{3}]}{[NO_{2}][SO_{2}]}](/tpl/images/0156/8615/e939f.png)
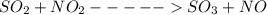
![Kc=3.80 = \frac{[1][1]}{[a-1][1.5]}](/tpl/images/0156/8615/9a395.png)