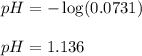Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 14:00
What mass of natural gas (ch4) must you burn to emit 276 kj of heat?
Answers: 1


Chemistry, 23.06.2019 01:00
What type of chemical bond is formed between two atoms of bromine 1. metallic 2. hydrogen 3. ionic 4. covalent
Answers: 1

Chemistry, 23.06.2019 04:00
Achemical reaction is done in the setup shown , resulting in a change of mass. what will happen if the same reaction is done in a sealed container that is placed on the electronic balance?
Answers: 2
You know the right answer?
In a titration of 47.41 ml of 0.3764 m ammonia with 0.3838 m aqueous nitric acid, what is the ph of...
Questions




















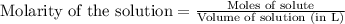
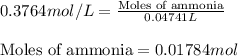
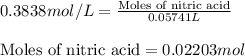
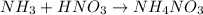
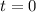 0.0178 0.022
0.0178 0.022![pH=-\log[H^+]](/tpl/images/0156/8638/cf945.png)
![[H^+]=\frac{0.0042mol}{0.05741L}=0.0731M](/tpl/images/0156/8638/dda8c.png)