
Chemistry, 01.08.2019 04:10 ramirezdolores
Determine the rate law and the value of k for the following reaction using the data provided. co(g) cl2(g) → cocl2(g)[co]i (m)[cl2]i(m)initial rate (m-1s-1)0.250.400.6960.250.801.970. 500.803.94a) rate

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 11:30
What is the main reason why some developing countries fear the increase the free trade policies around the world?
Answers: 2

Chemistry, 22.06.2019 13:30
If the concentration of phosphate in the cytosol is 2.0 mm and the concentration of phosphate in the surrounding fluid is 0.1 mm, how could the cell increase the concentration of phosphate in the cytosol? a) passive transportb) diffusionc) active transportd) osmosise) facilitated diffusion
Answers: 3


Chemistry, 23.06.2019 05:30
Awhite powder is added to a solution. the images show observations made before the powder is added, just after the powder has been added, and a little while later. (the liquid in the small beaker is phenol red solution.) what evidence shows that a chemical change has taken place?
Answers: 1
You know the right answer?
Determine the rate law and the value of k for the following reaction using the data provided. co(g)...
Questions


Mathematics, 18.03.2021 02:20



Mathematics, 18.03.2021 02:20

Biology, 18.03.2021 02:20


Mathematics, 18.03.2021 02:20

Mathematics, 18.03.2021 02:20


Mathematics, 18.03.2021 02:20



Mathematics, 18.03.2021 02:20

World Languages, 18.03.2021 02:20



Mathematics, 18.03.2021 02:20

Geography, 18.03.2021 02:20

![\text{Rate}=k[CO]^1[Cl_2]^{\frac{3}{2}}](/tpl/images/0156/8683/d3cd0.png) and value of 'k' is
and value of 'k' is 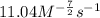
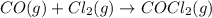
![\text{Rate}=k[CO]^a[Cl_2]^b](/tpl/images/0156/8683/d760d.png)
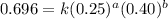 ....(1)
....(1)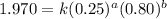 ....(2)
....(2)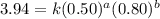 ....(3)
....(3)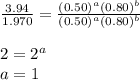
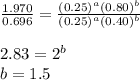
![0.696=k[0.25]^1[0.40]^{\frac{3}{2}}\\\\k=11.04M^{-\frac{7}{2}}s^{-1}](/tpl/images/0156/8683/569d9.png)


