Chemistry, 01.08.2019 04:20 arodriguez395
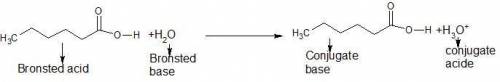
One of the substances that give wet goats and dirty gym socks their characteristic odors is hexanoic acid, ch3ch2ch2ch2ch2co2h, which is a monoprotic weak acid. write the formula for the conjugate base of this acid. write the equation for the reaction between this acid and water, and indicate the brønstedlowry acid and base for the forward reaction. (the acidic hydrogen atom is on the right side of the formula.)

Answers: 1


Another question on Chemistry



Chemistry, 23.06.2019 03:00
Can someone me out on this question for my national 5 chemistry homework
Answers: 1

Chemistry, 23.06.2019 09:00
The vapor pressure of water at 25.0°c is 23.8 torr. determine the mass of glucose (molar mass = 180 g/mol) needed to add to 500.0 g of water to change the vapor pressure to 22.8 torr.
Answers: 1
You know the right answer?
One of the substances that give wet goats and dirty gym socks their characteristic odors is hexanoic...
Questions



Mathematics, 08.02.2021 01:10

Mathematics, 08.02.2021 01:10


Advanced Placement (AP), 08.02.2021 01:10



Computers and Technology, 08.02.2021 01:10





Mathematics, 08.02.2021 01:10



Mathematics, 08.02.2021 01:10


Mathematics, 08.02.2021 01:10