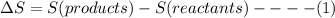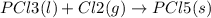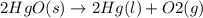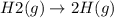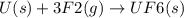Chemistry, 01.08.2019 05:10 nicole10perez
State whether the sign of the entropy change expected for each of the following processes will be positive or negative, and explain your predictions. (a) pcl3(l) + cl2(g) ⟶pcl5(s) (b) 2hgo(s) ⟶2hg(l) + o2(g) (c) h2(g) ⟶2h(g) (d) u(s) + 3f2(g) ⟶uf6(s)

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 20:30
12. complete each of the following word equations for synthesis reactions. a. sodium + oxygen -> b. magnesium + fluorine -> 13. complete and balance the equations for the decomposition reactions. a. hgo -> [with the triangle heat symbol above the arrow] b. h2o(l) -> [with "electricity" written above the arrow]
Answers: 1

Chemistry, 22.06.2019 01:00
What are the variables in gay-lussac’s law? pressure and volume pressure, temperature, and volume pressure and temperature volume, temperature, and moles of gas
Answers: 1

Chemistry, 22.06.2019 21:30
Isopropyl alcohol, (ch3)2choh, is a common solvent. determine the percent by mass of hydrogen in isopropyl alcohol. a) 6.71% h b) 13.4% h c) 25.0% h d) 53.3% h
Answers: 1

Chemistry, 23.06.2019 00:00
Total the mass on the syringe. record it in the correct row of the data table. kg done click and drag weights to change the pressure. click the syringe to zoom in and see the volume. intro
Answers: 3
You know the right answer?
State whether the sign of the entropy change expected for each of the following processes will be po...
Questions

Mathematics, 19.12.2019 05:31


Mathematics, 19.12.2019 05:31

Social Studies, 19.12.2019 05:31


Health, 19.12.2019 05:31


Mathematics, 19.12.2019 05:31

Mathematics, 19.12.2019 05:31



Mathematics, 19.12.2019 05:31




Mathematics, 19.12.2019 05:31