Chemistry, 01.08.2019 05:10 kayleefaithblair
At 25°c and constant pressure, carbon monoxide gas combines with oxygen gas to give carbon dioxide gas with the evolution of 10.1 kj per gram of carbon monoxide consumed. what is the value of δh for the reaction as represented by the equation below.

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 03:40
Chemical kinetics what was the rate of reaction in trial 3? choose the closest answer.
Answers: 3

Chemistry, 22.06.2019 07:40
The formation of a solid, also known as a is an indication of a chemical change. precipitate particulate particle powder
Answers: 3


Chemistry, 22.06.2019 12:10
Consider the reaction: n2(g) + o2(g) ⇄ 2no(g) kc = 0.10 at 2000oc starting with initial concentrations of 0.040 mol/l of n2 and 0.040 mol/l of o2, calculate the equilibrium concentration of no in mol/l how would this be done?
Answers: 3
You know the right answer?
At 25°c and constant pressure, carbon monoxide gas combines with oxygen gas to give carbon dioxide g...
Questions



Mathematics, 24.06.2020 01:01

World Languages, 24.06.2020 01:01

English, 24.06.2020 01:01




Mathematics, 24.06.2020 01:01

Mathematics, 24.06.2020 01:01



German, 24.06.2020 01:01







Mathematics, 24.06.2020 01:01

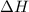 for the reaction is, -565.6 kJ
for the reaction is, -565.6 kJ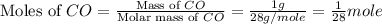
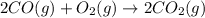
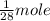 of CO release heat = 10.1 kJ
of CO release heat = 10.1 kJ