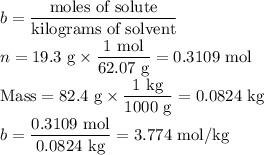
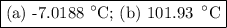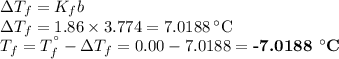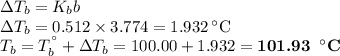An ethylene glycol solution contains 19.3g of ethylene glycol (c2h6o2) in 82.4ml of water.
(a)...

Chemistry, 01.08.2019 05:10 pricillagarcia2002
An ethylene glycol solution contains 19.3g of ethylene glycol (c2h6o2) in 82.4ml of water.
(a) compute the freezing point of the solution. (assume a density of 1.00 g/ml for water.)
(b)compute the boiling point of the solution. (assume a density of 1.00 g/ml for water.)
express your answer using five significant figures.

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 21:00
Consider the nuclear equation below. 239 > x + 4 he 94 2 what is x? 1.235 cm 96 2.243 u 92 3.235 u 92 4.243 cm 96
Answers: 2

Chemistry, 22.06.2019 10:00
Diffraction is when light is bent around obstructions. which of the these observation about clouds would indicate diffraction? a) after rain storms, you can sometimes see rainbows. b) clouds are white or gray and cannot be seen through. c) on a cloudy day, the temperature tends to be cooler than a sunny day. d) the edges of dark clouds appear lighter. this
Answers: 3

Chemistry, 22.06.2019 14:30
In water, a strong acid will break down into its component parts. a. completely b. partly c. never in water, a weak base will break down into its component parts. a. completely b. partly c. never
Answers: 2

Chemistry, 22.06.2019 22:00
Plz ill give u brainliest which of the following steps is not likely to take place during cellular respiration? a.oxygen combines with carbon of simple sugar. b. energy molecule transfers energy to cells. d. energy is used up.
Answers: 3
You know the right answer?
Questions

Mathematics, 08.12.2020 22:20



Mathematics, 08.12.2020 22:20

Mathematics, 08.12.2020 22:20

History, 08.12.2020 22:20


Mathematics, 08.12.2020 22:20

History, 08.12.2020 22:20


Biology, 08.12.2020 22:20

Social Studies, 08.12.2020 22:20



English, 08.12.2020 22:20


Mathematics, 08.12.2020 22:20


Mathematics, 08.12.2020 22:20

Computers and Technology, 08.12.2020 22:20