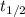Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 00:20
Use the gizmo to find the concentration of the mystery ch3cooh. use the titrant and indicator shown below perform the titration. what is the titrant volume? titrant analyte indicator titrant volume analyte concentration naoh ch3cooh phenophthalein select one: a. 20.0 ml b. 27.0 ml c. 30.0 ml d. 24.0 ml
Answers: 2

Chemistry, 22.06.2019 04:30
How much energy is made when a pice of wood burns. how do you know
Answers: 2

Chemistry, 22.06.2019 11:00
What is the molar mass of a gas that has density of 2.054 g/l
Answers: 2

You know the right answer?
The decomposition of nitrogen dioxide to nitrogen monoxide and oxygen gas is a second order process...
Questions


Mathematics, 18.09.2019 02:30

English, 18.09.2019 02:30

Mathematics, 18.09.2019 02:30


Mathematics, 18.09.2019 02:30

Biology, 18.09.2019 02:30


Biology, 18.09.2019 02:30



Mathematics, 18.09.2019 02:30


Chemistry, 18.09.2019 02:30




History, 18.09.2019 02:30

Chemistry, 18.09.2019 02:30

Social Studies, 18.09.2019 02:30

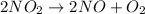
![t_{1/2} = \frac{1}{[A_{0}]k}](/tpl/images/0157/0306/7be8e.png) ....... (1)
....... (1)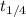 . So, expression for this will be as follows.
. So, expression for this will be as follows.![\frac{1}{k} [\frac{1}{[A]_{f}} - \frac{1}{[A_{0}]}]](/tpl/images/0157/0306/b672b.png) ...(2)
...(2)![[A]_{f}](/tpl/images/0157/0306/aeb4f.png) is the final concentration that is,
is the final concentration that is, ![\frac{[A]_{0}}{4}](/tpl/images/0157/0306/009c9.png) here and
here and ![[A]_{i}](/tpl/images/0157/0306/49e7e.png) is the initial concentration.
is the initial concentration.![\frac{1}{k} [\frac{4}{[A]_{0}} - \frac{1}{[A_{0}]}]](/tpl/images/0157/0306/9d389.png)
![\frac{3}{[A_{0}]k}](/tpl/images/0157/0306/df0b5.png) ...... (3)
...... (3)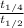 =
= ![\frac{3}{[A_{0}]k} \times [A_{0}]k](/tpl/images/0157/0306/a9d9d.png)