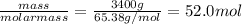Chemistry, 02.08.2019 19:10 abbeygrace13
Galvanized nails are iron nails that have been plated with zinc to prevent rusting. the relevant reaction is zn2+(aq)+2e−→zn(s) for a large batch of nails, a manufacturer needs to plate a total zinc mass of 3.40 kg on the surface to get adequate coverage. part a how many moles of zinc are in 3.40 kg of zinc? express your answer to three significant figures and include the appropriate units.

Answers: 1


Another question on Chemistry


Chemistry, 22.06.2019 15:30
Each of the following reactions is allowed to come to equilibrium and then the volume is changed as indicated. predict the effect (shift right, shift left, or no effect) of the indicated volume change. drag the appropriate items to their respective bins.co(g) + h2o(g) < => co2(g) + h2(g) (volume is decreased) pcl3(g) + cl2(g) < => pcl5(g) (volume is increased) caco3(s)< => cao(s) + co2(g) (volume is increased)
Answers: 1

Chemistry, 22.06.2019 16:30
For the reaction shown, calculate how many moles of no2 form when each of the following completely reacts. 2n2o5(g)→4no2(g)+o2(g) part a 1.0 mol n2o5 express your answer using two significant figures. nothing mol m o l request answer part b 5.4 mol n2o5 express your answer using two significant figures.
Answers: 2

Chemistry, 23.06.2019 03:30
The molar mass of nickel(ni) is 58.7 g/mol. how many moles are in an 88 gram sample of nickel?
Answers: 1
You know the right answer?
Galvanized nails are iron nails that have been plated with zinc to prevent rusting. the relevant rea...
Questions




Mathematics, 18.09.2021 19:30


History, 18.09.2021 19:30


Mathematics, 18.09.2021 19:30


Chemistry, 18.09.2021 19:30


Mathematics, 18.09.2021 19:30



English, 18.09.2021 19:30




Mathematics, 18.09.2021 19:30

History, 18.09.2021 19:30