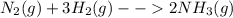Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 00:50
If a reactant was removed, did the new equilibrium system shift to make more reactants or more products?
Answers: 1

Chemistry, 22.06.2019 13:50
Amap that uses a range of colors and shading to represent the elevation, depth, or landscape of specific features on earth is a/an map.
Answers: 3

Chemistry, 22.06.2019 18:00
An object displaces 652 ml of water. the volume of the object is: 0.652 cm³ 6.52 cm³ 65.2 cm³ 652 cm³
Answers: 2

Chemistry, 23.06.2019 00:00
How many atoms or molecules are there in a mole of a substance?
Answers: 1
You know the right answer?
Nitrogen and hydrogen combine at a high temperature, in the presence of a catalyst, to produce ammon...
Questions

Mathematics, 04.10.2021 06:30


Biology, 04.10.2021 06:30



Physics, 04.10.2021 06:30


History, 04.10.2021 06:30

English, 04.10.2021 06:30



Mathematics, 04.10.2021 06:30


Mathematics, 04.10.2021 06:40

English, 04.10.2021 06:40

Mathematics, 04.10.2021 06:40

Physics, 04.10.2021 06:40



Mathematics, 04.10.2021 06:40