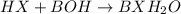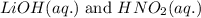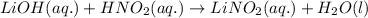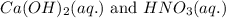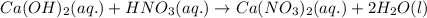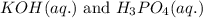Chemistry, 02.08.2019 22:20 Animallover100
Write the complete equation for the neutralization reactions that take place when the following water solutions are mixed. (if an acid has more than one acidic hydrogen, assume that there is enough base to remove all of them. assume that there is enough acid to neutralize all of the basic hydroxide ions.) a. lioh(aq) hno2(aq) b. co(oh)2(s) hno3(aq) c. h3po4(aq) koh(aq

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 09:40
Apiece of copper has a temperature of 75.6 0c. when the metal is placed in 100.0 grams of water at 19.1 0c, the temperature rises by 5.5 0c. what is the mass of the metal?
Answers: 1

Chemistry, 22.06.2019 19:00
Avolleyball player hit a ball with a mass of 0.25 kg. the average acceleration of the ball is 15.5 m/s². how much force did the volleyball player apply to the ball? 62.0 n 3.87 n 62.0 m/s² 3.87 m/s²
Answers: 2

Chemistry, 22.06.2019 21:30
Describe at least two advantages and two disadvantages of using hydropower as a source of energy.
Answers: 2

You know the right answer?
Write the complete equation for the neutralization reactions that take place when the following wate...
Questions



Mathematics, 05.12.2019 04:31

Mathematics, 05.12.2019 04:31


Health, 05.12.2019 04:31

History, 05.12.2019 04:31

Mathematics, 05.12.2019 04:31


Mathematics, 05.12.2019 04:31

Biology, 05.12.2019 04:31


Mathematics, 05.12.2019 04:31

History, 05.12.2019 04:31

Spanish, 05.12.2019 04:31

Mathematics, 05.12.2019 04:31

Biology, 05.12.2019 04:31


English, 05.12.2019 04:31