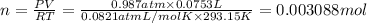Chemistry, 02.08.2019 22:20 shongmadi77
The reaction of solid aluminum with hydrochloric acid is used to make hydrogen gas in a laboratory experiment. the reaction is 2al(s) + 6hcl(aq) → 2alcl3(aq) + 3h2(g). the hydrogen gas is collected over water. how many moles of hydrogen gas were formed when 75.3 ml is collected at 20.0oc and 768.0 torr pressure? the vapor pressure of water at this temperature is 17.5 torr.

Answers: 1


Another question on Chemistry


Chemistry, 22.06.2019 09:20
What happened to the amount of carbon dioxide in the atmosphere from 2010–2017?
Answers: 1

Chemistry, 22.06.2019 11:30
Voltaic cells produce a positive overall charge. what does this indicate? a. the reaction is likely to be endothermic. b. the reaction is spontaneous. c. the reaction is not likely to occur. d. the reaction is not spontaneous.
Answers: 3

Chemistry, 22.06.2019 12:30
Acontrol during an experiment. might change remains constant does not exist does change
Answers: 1
You know the right answer?
The reaction of solid aluminum with hydrochloric acid is used to make hydrogen gas in a laboratory e...
Questions

Physics, 29.08.2021 22:50

Mathematics, 29.08.2021 22:50

Chemistry, 29.08.2021 22:50


Social Studies, 29.08.2021 22:50

Business, 29.08.2021 22:50

English, 29.08.2021 22:50

Mathematics, 29.08.2021 22:50

Chemistry, 29.08.2021 22:50




Medicine, 29.08.2021 22:50




Mathematics, 29.08.2021 22:50

Mathematics, 29.08.2021 22:50

Mathematics, 29.08.2021 22:50

Social Studies, 29.08.2021 22:50