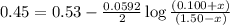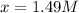Chemistry, 02.08.2019 22:30 meeeekmill
Avoltaic cell consists of a zn> zn2+ half-cell and a ni> ni2+ half-cell at 25 °c. the initial concentrations of ni2+ and zn2+ are 1.50 m and 0.100 m, respectively. a. what is the initial cell potential? b. what is the cell potential when the concentration of ni2+ has fallen to 0.500 m? c. what are the concentrations of ni2+ and zn2+ when the cell potential falls to 0.45 v?

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 05:30
What royal scientist used the 29th day of frozen vapor to encounter elements for mastering new culinary creations?
Answers: 1

Chemistry, 22.06.2019 18:10
Measurements that have similar values are: a. usually accurate b. sometimes accurate c. always accurate d. never accurate
Answers: 1


Chemistry, 23.06.2019 00:30
Five different substances are given to you to be dissolved in water. which substances are most likely to undergo dissolution in water? check all that apply. view available hint(s) check all that apply. sodium fluoride, naf octane, c8h18 propanol, ch3ch2ch2oh potassium iodide, ki benzene, c6h6
Answers: 1
You know the right answer?
Avoltaic cell consists of a zn> zn2+ half-cell and a ni> ni2+ half-cell at 25 °c. the initial...
Questions


Biology, 28.01.2020 21:55

History, 28.01.2020 21:55


English, 28.01.2020 21:55



Mathematics, 28.01.2020 21:55



Biology, 28.01.2020 21:55




History, 28.01.2020 21:55

Geography, 28.01.2020 21:55


Mathematics, 28.01.2020 21:55

History, 28.01.2020 21:55

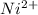 has fallen to 0.500 M is, 0.52 V
has fallen to 0.500 M is, 0.52 V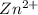 when the cell potential falls to 0.45 V are, 0.01 M and 1.59 M
when the cell potential falls to 0.45 V are, 0.01 M and 1.59 M![E^0_{[Ni^{2+}/Ni]}=-0.23V](/tpl/images/0163/3369/be6be.png)
![E^0_{[Zn^{2+}/Zn]}=-0.76V](/tpl/images/0163/3369/4cd18.png)
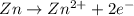
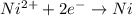
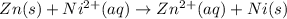
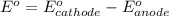
![E^o=E^o_{[Ni^{2+}/Ni]}-E^o_{[Zn^{2+}/Zn]}](/tpl/images/0163/3369/7d468.png)
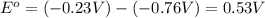
![E_{cell}=E^o_{cell}-\frac{0.0592}{n}\log \frac{[Zn^{2+}]}{[Ni^{2+}]}](/tpl/images/0163/3369/c02a9.png)
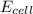 = emf of the cell = ?
= emf of the cell = ?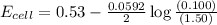
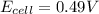
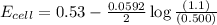
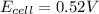
![E_{cell}=E^o_{cell}-\frac{0.0592}{n}\log \frac{[Zn^{2+}+x]}{[Ni^{2+}-x]}](/tpl/images/0163/3369/e92ff.png)